Abstract
Vibrotactile feedback (VTF) has been shown to improve balance performance in healthy people and people with vestibular disorders in a single-task experimental condition. It is unclear how age-related changes in balance affect the ability to use VTF and if there are different attentional requirements for old and young adults when using VTF. Twenty younger and 20 older subjects participated in this two-visit study to examine the effect of age, VTF, sensory condition, cognitive task, duration of time, and visit on postural and cognitive performance. Postural performance outcome measures included root mean square of center of pressure (COP) and trunk tilt, and cognitive performance was assessed using the reaction time (RT) from an auditory choice RT task. The results showed that compared with younger adults, older adults had an increase in COP in fixed platform conditions when using VTF, although they were able to reduce COP during sway-referenced platform conditions. Older adults also did not benefit fully from using VTF in their first session. The RTs for the secondary cognitive tasks increased significantly while using the VTF in both younger and older adults. Older adults had a larger increase compared with younger adults, suggesting that greater attentional demands were required in older adults when using VTF information. Future training protocols for VTF should take into consideration the effect of aging.
Keywords: vibrotactile feedback, dual task, postural sway, aging, reaction time, sensory substitution, balance
postural control is a perceptual motor process involving the collection and processing of sensory information and the execution of appropriate motor responses (Schmidt 1975). Sensory information from the visual, somatosensory, and vestibular systems contributes to the maintenance of human postural control (Dichgans and Diener 1989; Horak et al. 1990). Age-related declines in visual, somatosensory, and vestibular function may contribute to an increase in the risk of falling in older adults (Agrawal et al. 2010; Bergin et al. 1995; Lord et al. 1992). Changes in executive function and neuromuscular function are also related to increasing fall rates in older adults (de Rekeneire et al. 2003; Kearney et al. 2013). Falls in older adults not only impact personal health but also affect the person socially and economically (Davis et al. 2010a; Stevens et al. 2008).
Sensory substitution is a technique that uses a sensory modality to replace or augment another sensory modality (Bach-y-Rita et al. 1969). Various sensory substitution devices providing auditory, vibrotactile, and multimodal biofeedback have been proposed to counter age- and disease-related imbalance and to decrease the risk of falls (Dozza et al. 2007; Honegger et al. 2013; Nanhoe-Mahabier et al. 2012; Wall et al. 2009). Vibrotactile feedback (VTF) is one feedback modality that has been developed to provide individuals with balance problems with an external cue about how their body is moving in space (Wall et al. 2001). An inertial measurement unit (IMU), which is used to detect body motion; a processor; and a haptic display are typically included in a VTF system. Vibration cues are provided as feedback when a person's trunk or head exceeds a predefined, motion-based threshold. Several studies have validated the effect of VTF applied to the trunk on reducing postural sway in young, healthy subjects and people with vestibular deficits (Basta and Ernst 2011; Bechly et al. 2013; Dozza et al. 2007; Kentala et al. 2003; Lee et al. 2012; Sienko et al. 2008, 2010, 2012; Wall and Kentala 2005; Wall et al. 2009, 2001). VTF has also been demonstrated to reduce trunk tilt and improve gait performance in older adults (Haggerty et al. 2012; Wall et al. 2009). However, it has not been determined if the use of VTF to improve balance performance in older adults differs from that of younger adults.
Studies have suggested that postural control requires attention and is affected by age-related changes in attention (Mahboobin et al. 2007; Redfern et al. 2002; Shumway-Cook and Woollacott 2000). Dual postural-cognitive task paradigms have been used to study the relationship among attention, postural control, aging, and falls in the elderly. For example, older adults demonstrated slower reaction times (RTs) compared with younger adults on a secondary cognition task during dual postural-cognitive task conditions, which indicates an increase in attentional demands in older adults vs. younger adults (Brown et al. 1999; Prado et al. 2007; Rankin et al. 2000; Redfern et al. 2001, 2002; Shumway-Cook and Woollacott 2000). In addition, performance of walking while talking has been associated with a risk of falling in elderly persons (Lundin-Olsson et al. 1997; Verghese et al. 2002).
It can be argued that use of VTF represents another type of postural-cognitive task paradigm, since it requires the user to sense the vibration, process its meaning (e.g., direction and magnitude), and execute a motor response. If the use of VTF is a task that requires greater attentional resources, then the ability of older adults to use the VTF may be hindered compared with younger adults. Haggerty et al. (2012) have started to investigate this hypothesis in older adults by having them perform an auditory choice RT task (CRT) while using VTF. RTs increased when subjects received VTF compared with not using VTF, indicating that VTF requires greater attention. Nonetheless, older adult participants were still able to use the VTF to reduce root-mean-square (RMS) trunk tilt (Haggerty et al. 2012). However, their study did not characterize the effects of age-related changes in attention on balance performance. Another study that assessed postural-cognitive task performance while using multimodal feedback discovered that in contrast with younger adults, older adults were not able to use the feedback to reduce trunk sway while counting backwards and walking (Verhoeff et al. 2009).
The primary purpose of this study was to investigate the effect of age on postural and cognitive task performance while using VTF during various sensory balance conditions. A secondary aim was to assess the effect of using VTF within trials and over multiple visits. We hypothesized that older adults would have reduced postural performance [greater trunk tilt center of pressure (COP)] and reduced cognitive task performance (increased RT) compared with younger adults when using VTF and that performance would improve with greater use.
METHODS
Subjects.
Twenty healthy, younger adults (eight men and 12 women; mean age: 24.6, SD 2.4 yr; age range: 19–29 yr) and 20 healthy, older adults (10 men and 10 women; mean age: 75.4, SD 6.0 yr; age range: 65–84 yr) participated. Subjects were excluded during screening if they had neurologic or orthopedic disorders or were pregnant. In addition, subjects were excluded if they failed functional cognition and balance tests, with scores worse than 1.5 SD from the norm on the Repeatable Battery for the Assessment of Neuropsychological Status (Wilk et al. 2002), scores <19 on the Dynamic Gait Index (DGI) (Whitney et al. 2004), and Functional Gait Assessment scores that were <22 (Wrisley et al. 2004). In addition, subjects who had impaired sensation with the Semmes-Weinstein monofilament test (0.07 g) (Bell-Krotoski et al. 1995), abnormal age-corrected audiometric function, or binocular visual acuity with corrective lenses worse than 20/40 were excluded. The Institutional Review Board at the University of Pittsburgh approved the protocol.
Instrumentation.
The VTF system consisted of a belt, an IMU (Xsens Technologies B.V., Enschede, The Netherlands), eight vibrating tactors (C-2; Engineering Acoustics, Casselberry, FL), and a laptop computer. The belt was wrapped around the subject's waist, and two tactors were placed within the belt vertically, separated by 5 cm in each of the following locations: midline front, midline back, and right and left side of the body. The IMU was attached to the posterior of the belt at the level of the fourth lumbar vertebra. The IMU recorded angular velocity, linear acceleration, and magnetic field, from which the subject's trunk's angular position from vertical (i.e., trunk tilt) and angular velocity in the anteroposterior (AP) and mediolateral (ML) directions was estimated using manufacturer-provided functions in the software development kit. Static accuracy of the pitch-and-roll measurements, corresponding to tilt in the AP and ML directions, is 0.5°, and the angular velocity accuracy is 0.1°/s at a frequency of 0.25 Hz, which is typical for postural sway applications (Xsens Technologies B.V. 2014). VTF was provided when the proportional-plus-derivative feedback control signal—equal to the trunk angular position value (in degrees), plus 0.5 (in seconds), times the trunk angular velocity (degrees/second) (Goodworth et al. 2009; Sienko et al. 2008)—exceeded defined thresholds. Because this control signal incorporated velocity as well as position error terms, it effectively reduced the tactor activation threshold, theoretically enabling the subjects to quicken their response. The threshold of the lower-row tactors was set to 1.5° anteriorly, 0.5° posteriorly, and 0.5° to the right and left. The threshold of the upper-row tactors was set to 3° anteriorly, 1.5° posteriorly, and 1.5° to the right and left. The limits of stability are larger in the anterior direction compared with the posterior direction, so a larger threshold for anterior postural sway was set (Winter et al. 1996). “The nearest neighbor” principle was used in the feedback algorithm that activated only one tactor at a time by determining which direction had the greatest control signal value (Sienko et al. 2008). Tactor vibrations were at 250 Hz. Subjects were barefoot and wore a thin, standard shirt so that the vibrotactile cues could be sensed easily.
A computerized dynamic posturography platform (EquiTest; NeuroCom, Clackamas, OR) was used to record the COP. The EquiTest also provided sway referencing (SR) in the sagittal plane about the ankle joints by estimating the body pitch from the AP COP.
A secondary attention task was delivered by a customized program (LabVIEW; National Instruments, Austin, TX), providing an auditory CRT. The auditory CRT stimuli consisted of 560 and 980 Hz tones, transmitted through a set of earphones (E-A-RTONE). The tones were played at 80 dB for 250 ms and repeated every 2–6 s during a 2-min period. With the use of one microswitch button in each hand, the subject pressed the button in the dominant hand for a high-pitch tone and the nondominant hand for a low-pitch tone. Twenty-five to 29 stimuli were presented in each trial. The onset of the switch activation relative to the stimulus was recorded with a temporal resolution of 1 ms.
Experimental procedure.
Each subject completed three study visits, including one preliminary visit and two experimental visits. The average number of days between the two experimental visits for the young group was 6 (SD 3) days and for the older group was 6 (SD 4) days. A preliminary visit was used for screening and training the subject. The subject was briefly trained to perform the CRT tasks, use the VTF, and perform the CRT tasks while using the VTF. Five sensory integration conditions were used in the VTF training session: standing on a fixed platform with eyes open in light (Fixed/EO), standing on a fixed platform with eyes open in dark (Fixed/EOD), standing on a SR platform with EO (SR/EO), standing on a SR platform with EOD (SR/EOD), and standing on a SR platform with EO while performing the CRT tasks. The subjects were instructed to stand comfortably and to reduce the vibration as much as possible by moving in the opposite direction. Darkened goggles were used during the EOD condition to minimize visual reference cues. Each training condition lasted for 120 s. During the experimental visits 1 and 2, a short training session involving multiple training trials was held before the first experimental test. The 1-min training trials included one trial of the CRT task and five different sensory-integration conditions that were described above. Then, during each visit, a total of 16, 2-min experimental tests were performed, including all combinations of VTF on/off, CRT task on/off, and the sensory conditions (Fixed/EO, Fixed/EOD, SR/EO, and SR/EOD). The subjects performed the experimental conditions in random order during both of the experimental visits.
Outcome measures.
The postural performance measures were the trunk-tilt deviation from vertical and the COP in the AP and ML directions. To investigate within-trial performance, we divided the 120 s of data into four periods (period 1: 1–30 s; period 2: 31–60 s; period 3: 61–90 s; period 4: 91–120 s) (Carroll and Freedman 1993; O'Connor et al. 2008). The RMS of trunk tilt and RMS COP were computed after subtracting the mean value, via a custom MATLAB (MathWorks, Natick, MA) program. However, because the SR platform only moved in the AP direction, ML trunk tilt and COP were not included in the data analysis. The IMU data were only recorded during the trials with VTF so that the trunk tilt was only recorded in eight out of 16 trials. The COP was recorded during all of the 16 trials.
Cognitive task performance was assessed using the median RT, calculated for each of the eight trials with the CRT task. The first RT response was not included in the median calculation, because the subjects usually responded with an increased latency. The median RT was used to assess the influence of VTF, sensory condition, and between-visit factors on attention in the younger and older groups.
Statistical analysis.
A repeated-measures ANOVA was conducted to investigate the aims. A secondary analysis showed that whereas there was an interaction between platform condition and vision conditions on the RMS COP, this effect did not appear in any other higher-order interactions with any of the other factors. Consequently, we applied a simpler model using sensory condition (Condition) with four levels (Fixed/SR platform × EO/EOD) instead of including the platform and vision factors. The effects of Age, Period, Visit, CRT, and Condition variables were tested with the RMS trunk-tilt data, and the effects of Age, Period, Visit, VTF, CRT, and Condition variables were tested with the RMS COP. The postural performance data (RMS trunk tilt and RMS COP) were logarithmically transformed to meet the assumption of normality of repeated-measures ANOVA. A Bonferroni correction was applied if post hoc analysis was needed for the Condition and Period variables. The highest-order interactions considered were three-way interactions. Likewise, we investigated the effect of Age, Visit, VTF, and Condition on the median RT. All statistical analyses were performed using IBM SPSS Statistics, Version 19 (IBM, Armonk, NY). A significance level of α = 0.05 was used.
RESULTS
Postural performance.
The repeated-measures ANOVA revealed numerous, significant main effects and interactions on RMS COP (Table 1) and RMS trunk tilt (Table 2). Significant main effects included Age, Condition, Period, and Visit on RMS COP and RMS trunk tilt. Over all conditions, older adults had ∼33% greater RMS COP and 58% greater RMS trunk tilt than younger adults (P < 0.001). The sensory condition had a dramatic effect on the magnitude of RMS COP and RMS trunk tilt, increasing by more than a factor of three from the Fixed/EO condition to the SR/EOD condition (P < 0.001). A significant Period effect was observed (P < 0.001), which was due to greater RMS COP in the initial 30 s compared with the final 90 s and greater RMS trunk tilt in the last 30 s compared with the middle 60 s. There was a modest but significant Visit effect with reduced RMS COP (−4%) and RMS trunk tilt (−15%) during experimental visit 2 compared with experimental visit 1 (P < 0.005). Unexpectedly, there was not a significant reduction of RMS COP when VTF was used, due to interactions between VTF and other factors (as described below). The secondary CRT task did not significantly increase RMS COP or RMS trunk tilt. Furthermore, CRT did not appear in any higher-order interactions.
Table 1.
Effects of age, sensory condition, vibrotactile feedback (VTF), performance of auditory choice reaction time (CRT) tasks, period, and visit on the root-mean-square of the anterior-posterior center of pressure (RMS COP)
| Main Effects | RMS COP (Mean ± SD) | F and P Values | Interactions | F and P Values |
|---|---|---|---|---|
| Age |
|
F1,38 = 19.6, P < 0.001 |
|
|
| Condition* |
|
F1.9,71.2 = 564.1, P < 0.001 | ||
| VTF |
|
F1,38 = 0.4, P = 0.55 |
|
|
| CRT |
|
F1,38 = 3.8, P = 0.058 | ||
| Period† |
|
F2.3,88.2 = 26.2, P < 0.001 |
|
|
| Visit |
|
F1,38 = 8.8, P = 0.005 |
Platform conditions: fixed platform (Fixed) and sway-referenced platform (SR). Light conditions: eyes open (EO) and eyes open in the dark (EOD).
Post hoc test for Condition: all conditions were significantly different; P < 0.001.
Post hoc test for Period: period 1, significantly greater than periods 2–4; P < 0.001.
Table 2.
Effects of age, sensory condition, performance of auditory CRT task, period, and visit on the RMS of the anterior-posterior trunk tilt (RMS trunk tilt)
| Main Effects | RMS Trunk Tilt, ° (Mean ± SD) | F and P Values | Interactions | F and P Values |
|---|---|---|---|---|
| Age |
|
F1,38 = 14.5, P < 0.001 |
|
|
| Condition*† |
|
F2.4,85.2 = 284.2, P < 0.001 | ||
| CRT |
|
F1,36 = 0.2, P = 0.68 | ||
| Period |
|
F2.1,75.1 = 8.3, P < 0.001 | ||
| Visit |
|
F1,36 = 10.6, P = 0.002 |
Post hoc test for Condition: all conditions were significantly different; P < 0.001.
Post hoc test for Period: period 4 significantly greater than periods 2 and 3; P < 0.001.
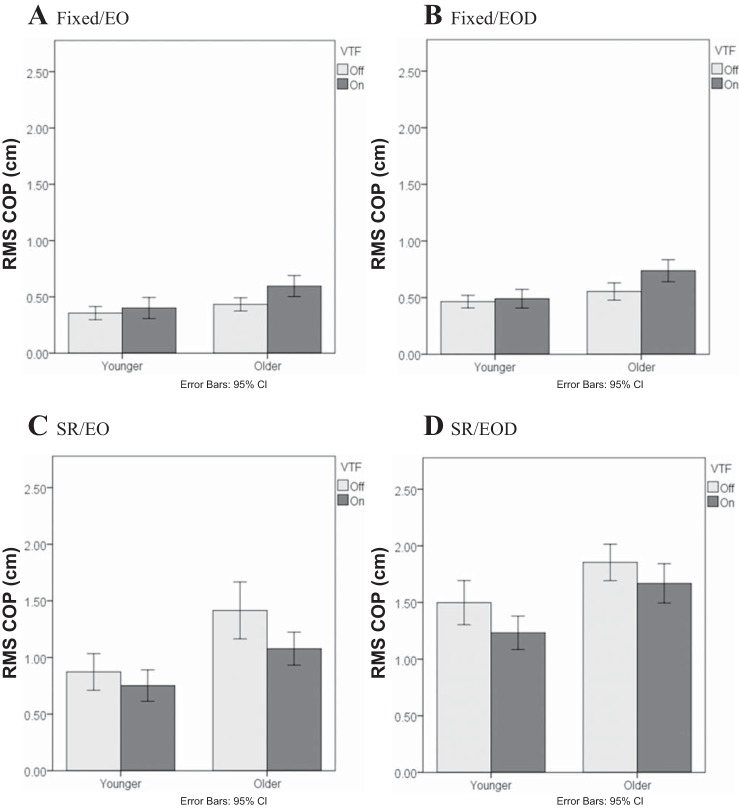
Evidence of an age effect on ability to use VTF was revealed in significant higher-order interactions that were discovered for the RMS COP data. First, there was a significant three-way interaction of Age-VTF-Condition (P < 0.001; Fig. 1). Specifically, during the fixed platform conditions (Fig. 1, A and B), greater RMS COP was observed when VTF was provided to older adults (P < 0.001), whereas there was no change in RMS COP during VTF in younger adults (P > 0.13). In contrast, during the SR platform conditions (Fig. 1, C and D), application of VTF reduced RMS COP in both older and younger adults (P < 0.022). Thus the use of VTF had an unexpected influence on COP in older adults on the fixed platform trials.
Fig. 1.
Effect of vibrotactile feedback (VTF)-Age-Condition interaction on the root-mean-square of the anterior-posterior center of pressure (RMS COP). Light conditions: eyes open (EO) and eyes open in the dark (EOD). Platform conditions: fixed platform (Fixed) and sway-referenced platform (SR). CI, confidence interval.
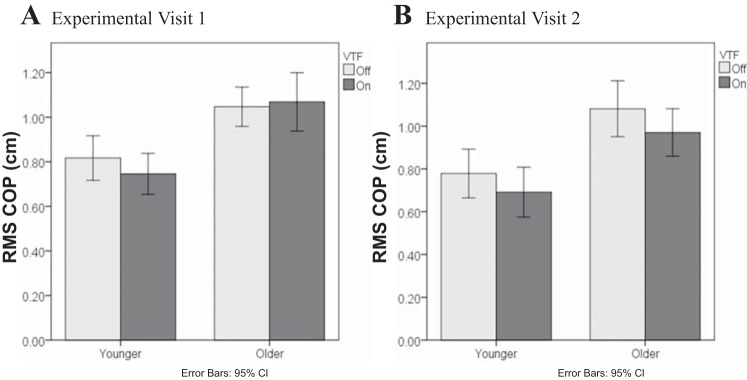
A significant Age-VTF-Visit interaction also demonstrated a difference in older adults' ability to use VTF (P = 0.018; Fig. 2). Whereas the reduction in RMS COP with VTF was consistent across visits in younger adults (P = 0.46), older adults had no improvement in RMS COP with VTF on visit 1 but a significant improvement in COP with VTF on visit 2 (P = 0.003).
Fig. 2.
Effect of VTF-Age-Visit interaction on the RMS COP.
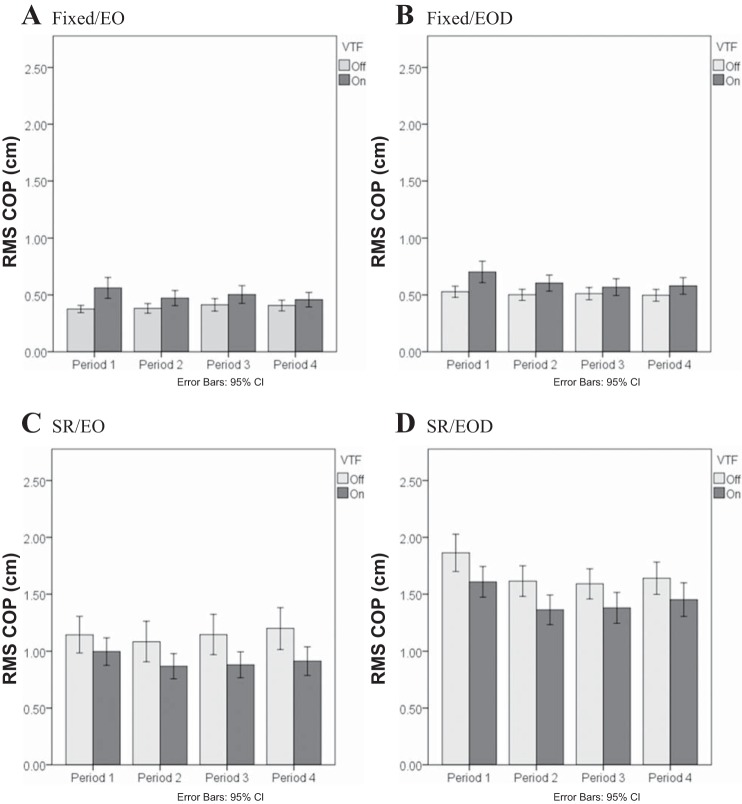
The final three-way interaction from the COP analysis consisted of the factors Period, VTF, and Condition (P = 0.02; Fig. 3). The three-way interaction illustrates that during the fixed platform conditions (Fig. 3, A and B), RMS COP was relatively level across all four periods when there was no VTF. When VTF was available, although the overall RMS COP increased compared with no VTF, there was a reduction of RMS COP during periods 2, 3, and 4 compared with period 1 (P < 0.007). During the SR platform conditions (Fig. 3, C and D), the reduction in RMS COP during the VTF was relatively stable across all periods.
Fig. 3.
Effect of Period-VTF-Condition interaction on the RMS COP. Light conditions: EO and EOD. Platform conditions: Fixed and SR. Period 1: 1–30 s; Period 2: 31–60 s; Period 3: 61–90 s; Period 4: 91–120 s.
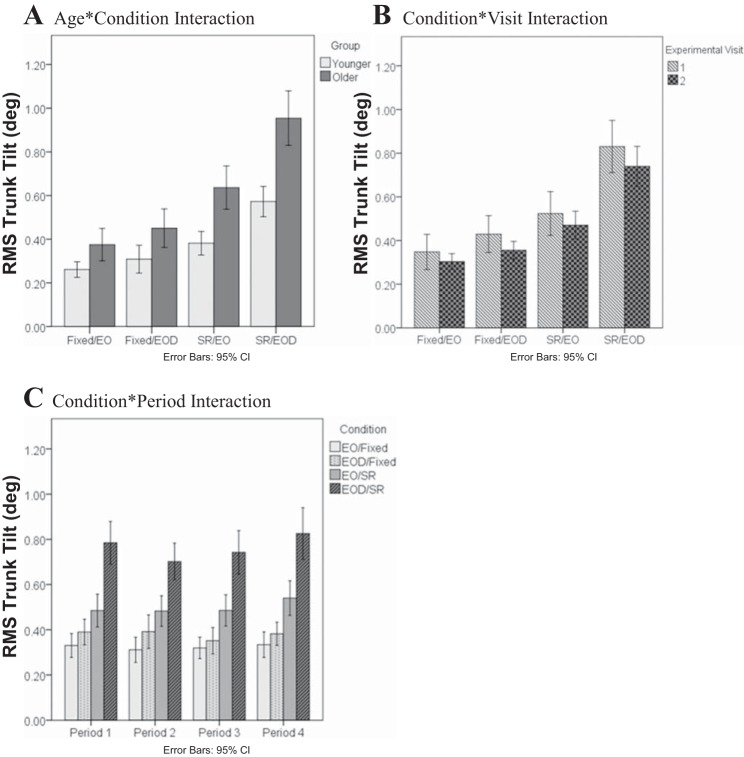
There were significant interactions among the factors on the RMS trunk tilt (Table 2 and Fig. 4). Foremost was the significant Age-Condition interaction (P = 0.028), which revealed that during the conditions when VTF was available, older adults had an impaired ability to use the VTF to control their trunk tilt compared with the younger adults, as the sensory conditions became more difficult (Fig. 4A). Although the Condition-Visit interaction (P = 0.049) was found, the post hoc analysis did not reveal any statistical difference between different visits among all conditions (Fig. 4B). The Condition-Period interaction (P = 0.021) illustrated that in the Fixed/EOD condition, there was a decrease in RMS trunk tilt in period 3 compared with the other periods. In the SR/EO condition, period 4 had the largest RMS trunk tilt. In the SR/EOD condition, there was a decrease in RMS trunk tilt from period 1 to period 2 and then an increase from period 2 to period 4 (Fig. 4C).
Fig. 4.
Age-Condition (A), Condition-Visit (B), and Condition-Period (C) interactions on the RMS of the anterior-posterior trunk tilt (RMS Trunk Tilt) when VTF was applied.
Cognitive task performance.
A repeated-measures ANOVA of the median RT from each trial demonstrated significant main effects of Age, Condition, and VTF (P < 0.001; Table 3). The RTs of older adults were slower than younger adults by 109 ms (+27%). RTs increased as the challenge of the sensory condition increased. In particular, the SR/EOD condition produced RTs significantly greater than all of the other conditions, and the SR/EO condition resulted in greater RTs compared with the Fixed/EO condition. When VTF was used, the RTs increased ∼69 ms (+16%) compared with when VTF was not used.
Table 3.
Effects of age, sensory condition, VTF, and visit on the median reaction time during performance of an auditory CRT task
| Main Effects | Reaction Time, ms (Mean ± SD) | F and P Values | Interactions | F and P Values |
|---|---|---|---|---|
| Age |
|
F1,38 = 14.5, P < 0.001 |
|
|
| Condition*† |
|
F3,114 = 29.1, P < 0.001 | ||
| VTF |
|
F1,38 = 80.2, P < 0.001 | ||
| Visit |
|
F1,38 = 2.4, P = 0.13 |
Platform conditions: Fixed and SR. Light conditions: EO and EOD.
Post hoc test for Condition: SR/EOD significantly greater than Fixed/EO, Fixed/EOD, and SR/EO; P < 0.001.
Post hoc test for Condition: SR/EO significantly greater than Fixed/EO; P = 0.006.
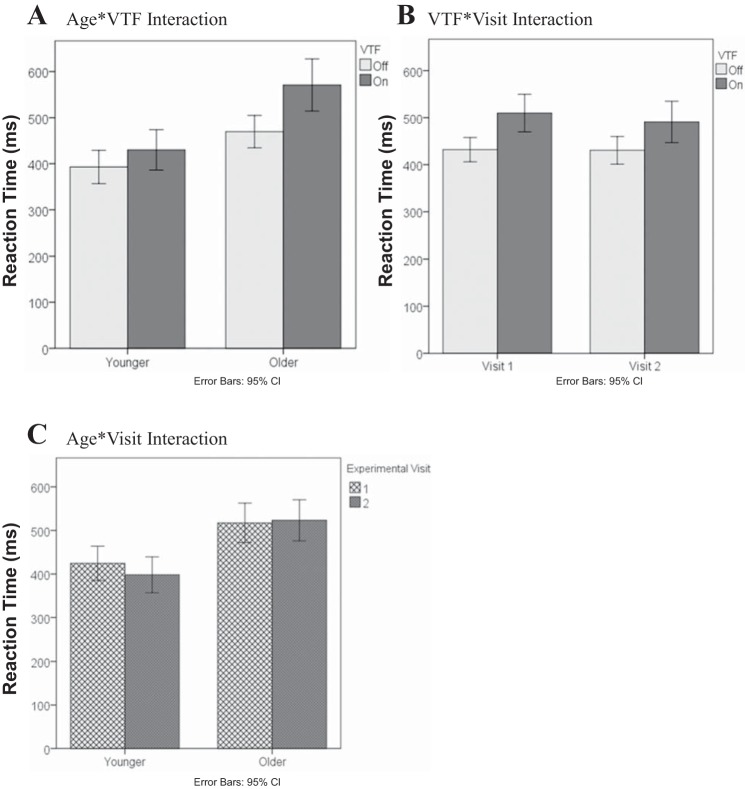
In addition, there were three significant two-way interactions, as shown in Fig. 5. The Age-VTF interaction (Fig. 5A) demonstrated that the increase in RTs during VTF was greater in older adults compared with younger adults (101 ms vs. 37 ms, P < 0.001). The VTF-Visit interaction (Fig. 5B) showed that the increase in RTs during VTF was greater in the first vs. the second visit (78 ms vs. 60 ms, P = 0.01). Finally, the Age-Visit interaction (Fig. 5C) indicated that younger adults had faster RTs on the second-study visit, whereas the RTs of the older group were essentially the same on both visits (−26 ms vs. +6 ms, P = 0.018).
Fig. 5.
Effect of Age-VTF (A), VTF-Visit (B), and Age-Visit (C) interactions on median reaction time.
DISCUSSION
Our primary hypothesis was that older adults would have reduced postural and cognitive task performance compared with younger adults when using VTF. In accordance, older adults demonstrated a worse performance in the following ways. First, the Age-VTF-Condition interaction demonstrated that COP increased significantly when VTF was used by older adults during fixed platform conditions (Fixed/EO: +37.4%; Fixed/EOD: +33.1%), whereas COP changed minimally in younger subjects (Fixed/EO: +12.6%; Fixed/EOD: +6%; Fig. 1). Increased COP indicates that greater ankle torque was needed to control the body sway (Winter et al. 1998). Second, as shown by the Age-VTF-Visit interaction (Fig. 2), older adults did not reduce COP in the first visit, indicating that they needed additional training to use the feedback appropriately. Furthermore, the results demonstrated that during the trials when trunk tilt was measured (i.e., the VTF on trials), older adults had greater increases in trunk tilt as the sensory conditions became more difficult compared with younger adults (Fig. 4). Finally, older adults had worse cognitive task performance than younger adults when VTF was used, as revealed by the Age-VTF interaction on RT (Fig. 5).
Postural performance.
Typically, the COP, reflective of ankle-torque generation, is considered to be the regulator of body center of mass (COM) or center of gravity (COG) movement, represented by trunk tilt during quiet standing (Winter et al. 1998). Winter (1995) showed that during quiet standing, body movements can be approximated by an inverted pendulum model, in which the horizontal acceleration of COM is proportional to the difference between COP and COG. Consequently, COG and COP are highly correlated when the acceleration is small. Thus it was no surprise that the results of both postural performance measures were consistent in showing significant main effects of Age, Condition, and Visit.
The RMS COP data demonstrated that younger and older subjects responded differently to VTF under various sensory integration tasks. When the platform was fixed, RMS COP increased when VTF was used by older adults during the first visit. However, when the platform was SR, RMS COP decreased similarly in both younger and older adults during VTF. These results suggest the use of different postural strategies between younger and older adults during the fixed platform condition while VTF was provided, and we speculate that older adults used a hip strategy to a greater extent during their first visit. Although the lack of kinematic data precludes confirmation of this postulation, Speers et al. (1998) have shown that the use of a hip strategy to maintain balance during a fixed platform condition in astronauts postspaceflight significantly increased sway compared with prespaceflight. It is also possible that VTF provided a type of perturbation and/or elicited an overcorrection as the older adults were still learning to use the VTF effectively during visit 1. If the older adults attempted to make postural corrections during this condition in response to the vibrotactile cues, then they may have done so by repositioning their trunk (i.e., larger corrections and therefore larger changes in COP) as opposed to initiating a corrective response using their ankles (i.e., smaller corrections and therefore smaller changes in COP). This strategy may have resulted in “overcorrections.” As their abilities to use the feedback in a controlled manner improved, as shown by the reduction in COP during visit 2, older subjects no longer demonstrated an increase in COP during the fixed platform conditions.
Several previous studies have documented the ability of older adults to use various modalities of feedback to control their standing balance and walking (Allum et al. 2011; Verhoeff et al. 2009; Wall et al. 2009). In these studies, training periods lasted from 20 min (Haggerty et al. 2012; Wall et al. 2009) to six visits over 2 wk (Allum et al. 2011). With the use of multimodal feedback, older adults were able to reduce trunk tilt while standing on level surfaces and foam and tandem walking, with eyes open or closed (Allum et al. 2011; Davis et al. 2010b). Likewise, with the use of VTF, older adults reduced trunk tilt during normal and semitandem stance with eyes open and closed (Haggerty et al. 2012) and improved their DGI scores (Wall et al. 2009). Two of the cited studies compared responses of older adults with younger adults. Consistent with our results, younger adults, but not older adults, were able to reduce their trunk tilt when a secondary arithmetic task was performed (Verhoeff et al. 2009). However, in contrast with our study, Davis et al. (2010b) demonstrated that the reduction in trunk tilt was not different between older and younger adults in most conditions. However, older subjects had a greater reduction in trunk tilt than younger subjects during tandem walking (Davis et al. 2010b). Thus whereas Davis et al. (2010b) showed that older adults have the ability to use feedback to reduce trunk tilt to the same degree as younger adults, there may be some limitations to this ability that depend on secondary cognitive engagement (Verhoeff et al. 2009), sensory environment, and training, as shown in this study.
We expected that the addition of a secondary cognitive task (i.e., auditory CRT) would negatively influence the amount of reduction in trunk tilt provided by VTF, especially in older adults. However, we failed to detect a significant main effect of the CRT on the postural performance measures, independent of the state of VTF, nor did we find an interaction between the CRT and Age. Likewise, Haggerty et al. (2012) did not find that performing a concurrent auditory CRT prevented older adults from reducing their trunk tilt when using VTF. These results corresponded with the study by Redfern et al. (2002), which found that COP was unchanged by the presence of the RT task. In our experiment, the posture-first principle may explain the negligible effect of the secondary cognitive task on COP (Lajoie et al. 1993; Shumway-Cook et al. 1997). In contrast, Verhoeff et al. (2009) reported that older adults were not able to use multimodal feedback to reduce trunk tilt and velocity while walking and counting backwards. Thus the ability to use VTF may depend on the type of primary or secondary task or other factors not elucidated.
An unresolved issue is the duration of time over which VTF is effective at reducing sway. We found that older adults did not see a benefit in their first experimental visit. It has been proposed that people with balance problems will wear VTF systems for the purpose of balance training or as a balance aid. In many of the previous research studies, the duration of using VTF was <1 min (Kentala et al. 2003; Sienko et al. 2012; Wall 2010; Wall et al. 2001). However, given that dynamic reweighting of sensory inputs to postural control can occur (Peterka and Loughlin 2004) and that VTF may be used for training over longer time spans, it is necessary to evaluate in future studies if responses to VTF change over longer periods of time.
Cognitive task performance.
The secondary cognitive task resulted in longer RTs in older adults compared with younger adults, consistent with previous studies (Redfern et al. 2002; Shumway-Cook and Woollacott 2000; Shumway-Cook et al. 1997). Furthermore, the use of VTF required additional attention during the sensory integration conditions, confirming the results of Haggerty et al. (2012). However, our data also suggested that the attention requirement in using VTF was greater in older adults than younger adults. Specifically, RTs increased by 101 ms (22%) in older adults and 37 ms (9%) in younger adults when VTF was present. The increase in RT is significant and suggests that more attentional resources are needed (Pashler 1998). The increase in attention needed to use VTF indicates that some older adults who have executive dysfunction may not be good candidates for using VTF.
Limitations.
Several study limitations were identified when we tried to interpret our data. We did not measure segmental body movement and therefore were not able to quantify differences in postural control strategies that may have been present between young and older adults. In addition, we did not assess trunk tilt during the conditions when VTF was not used. As a result, we were not able to assess the effect of VTF on trunk tilt and how VTF may have influenced COP and trunk tilt differently.
Conclusion.
Our data suggest that younger and older adults use VTF differently, depending on the underlying sensory conditions and amount of training. Although the use of VTF required more attention, older adults were able to use VTF to reduce COP and trunk tilt in SR platform conditions. The design of the optimal training protocol for VTF should take these factors into consideration.
GRANTS
This work was supported by the National Science Foundation (Grant no. 0846471 to K. H. Sienko) and the National Institute on Deafness and Other Communication Disorders (Grant no. 5R21-DC-012410-02 to K. H. Sienko and S. L. Whitney).
DISCLOSURES
Any opinions, findings, conclusions, or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation and U.S. National Institutes of Health. All authors reported no conflict of interest.
AUTHOR CONTRIBUTIONS
C.-C.L., S.L.W., P.J.L., J.M.F., M.S.R., K.H.S., and P.J.S. conception and design of research; C.-C.L. performed experiments; C.-C.L. analyzed data; C.-C.L., S.L.W., P.J.L., J.M.F., M.S.R., K.H.S., and P.J.S. interpreted results of experiments; C.-C.L. prepared figures; C.-C.L. drafted manuscript; C.-C.L., S.L.W., P.J.L., J.M.F., M.S.R., K.H.S., and P.J.S. edited and revised manuscript; C.-C.L., S.L.W., P.J.L., J.M.F., M.S.R., K.H.S., and P.J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors give special thanks to Dr. Beom-Chen Lee for his contributions to the development of the VTF device used in this study. The authors also thank Anita Lieb and Susan Strelinski for their help on this project.
REFERENCES
- Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Diabetes, vestibular dysfunction, and falls: analyses from the National Health and Nutrition Examination Survey. Otol Neurotol 31: 1445–1450, 2010. [DOI] [PubMed] [Google Scholar]
- Allum JH, Carpenter MG, Horslen BC, Davis JR, Honegger F, Tang KS, Kessler P. Improving impaired balance function: real-time versus carry-over effects of prosthetic feedback. Conf Proc IEEE Eng Med Biol Soc 2011: 1314–1318, 2011. [DOI] [PubMed] [Google Scholar]
- Bach-y-Rita P, Collins CC, Saunders FA, White B, Scadden L. Vision substitution by tactile image projection. Nature 221: 963–964, 1969. [DOI] [PubMed] [Google Scholar]
- Basta D, Ernst A. [Vibrotactile neurofeedback training with the Vertiguard®-RT-system. A placebo-controlled double-blinded pilot study on vestibular rehabilitation]. HNO 59: 1005–1011, 2011. [DOI] [PubMed] [Google Scholar]
- Bechly KE, Carender WJ, Myles JD, Sienko KH. Determining the preferred modality for real-time biofeedback during balance training. Gait Posture 37: 391–396, 2013. [DOI] [PubMed] [Google Scholar]
- Bell-Krotoski JA, Fess EE, Figarola JH, Hiltz D. Threshold detection and Semmes-Weinstein monofilaments. J Hand Ther 8: 155–162, 1995. [DOI] [PubMed] [Google Scholar]
- Bergin PS, Bronstein AM, Murray NM, Sancovic S, Zeppenfeld DK. Body sway and vibration perception thresholds in normal aging and in patients with polyneuropathy. J Neurol Neurosurg Psychiatry 58: 335–340, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LA, Shumway-Cook A, Woollacott MH. Attentional demands and postural recovery: the effects of aging. J Gerontol A Biol Sci Med Sci 54: M165–M171, 1999. [DOI] [PubMed] [Google Scholar]
- Carroll JP, Freedman W. Nonstationary properties of postural sway. J Biomech 26: 409–416, 1993. [DOI] [PubMed] [Google Scholar]
- Davis JC, Robertson MC, Ashe MC, Liu-Ambrose T, Khan KM, Marra CA. International comparison of cost of falls in older adults living in the community: a systematic review. Osteoporos Int 21: 1295–1306, 2010a. [DOI] [PubMed] [Google Scholar]
- Davis JR, Carpenter MG, Tschanz R, Meyes S, Debrunner D, Burger J, Allum JH. Trunk sway reductions in young and older adults using multi-modal biofeedback. Gait Posture 31: 465–472, 2010b. [DOI] [PubMed] [Google Scholar]
- de Rekeneire N, Visser M, Peila R, Nevitt MC, Cauley JA, Tylavsky FA, Simonsick EM, Harris TB. Is a fall just a fall: correlates of falling in healthy older persons. The Health, Aging and Body Composition Study. J Am Geriatr Soc 51: 841–846, 2003. [DOI] [PubMed] [Google Scholar]
- Dichgans J, Diener HC. The contribution of vestibulo-spinal mechanisms to the maintenance of human upright posture. Acta Otolaryngol 107: 338–345, 1989. [DOI] [PubMed] [Google Scholar]
- Dozza M, Wall C 3rd, Peterka RJ, Chiari L, Horak FB. Effects of practicing tandem gait with and without vibrotactile biofeedback in subjects with unilateral vestibular loss. J Vestib Res 17: 195–204, 2007. [PMC free article] [PubMed] [Google Scholar]
- Goodworth AD, Wall C 3rd, Peterka RJ. Influence of feedback parameters on performance of a vibrotactile balance prosthesis. IEEE Trans Neural Syst Rehabil Eng 17: 397–408, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty S, Jiang LT, Galecki A, Sienko KH. Effects of biofeedback on secondary-task response time and postural stability in older adults. Gait Posture 35: 523–528, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger F, Hillebrandt IM, van den Elzen NG, Tang KS, Allum JH. The effect of prosthetic feedback on the strategies and synergies used by vestibular loss subjects to control stance. J Neuroeng Rehabil 10: 115, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak FB, Nashner LM, Diener HC. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res 82: 167–177, 1990. [DOI] [PubMed] [Google Scholar]
- Kearney FC, Harwood RH, Gladman JR, Lincoln N, Masud T. The relationship between executive function and falls and gait abnormalities in older adults: a systematic review. Dement Geriatr Cogn Disord 36: 20–35, 2013. [DOI] [PubMed] [Google Scholar]
- Kentala E, Vivas J, Wall C 3rd.. Reduction of postural sway by use of a vibrotactile balance prosthesis prototype in subjects with vestibular deficits. Ann Otol Rhinol Laryngol 112: 404–409, 2003. [DOI] [PubMed] [Google Scholar]
- Lajoie Y, Teasdale N, Bard C, Fleury M. Attentional demands for static and dynamic equilibrium. Exp Brain Res 97: 139–144, 1993. [DOI] [PubMed] [Google Scholar]
- Lee BC, Kim J, Chen S, Sienko KH. Cell phone based balance trainer. J Neuroeng Rehabil 9: 10, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SR, McLean D, Stathers G. Physiological factors associated with injurious falls in older people living in the community. Gerontology 38: 338–346, 1992. [DOI] [PubMed] [Google Scholar]
- Lundin-Olsson L, Nyberg L, Gustafson Y. “Stops walking when talking” as a predictor of falls in elderly people. Lancet 349: 617, 1997. [DOI] [PubMed] [Google Scholar]
- Mahboobin A, Loughlin PJ, Redfern MS. A model-based approach to attention and sensory integration in postural control of older adults. Neurosci Lett 429: 147–151, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanhoe-Mahabier W, Allum JH, Pasman EP, Overeem S, Bloem BR. The effects of vibrotactile biofeedback training on trunk sway in Parkinson's disease patients. Parkinsonism Relat Disord 18: 1017–1021, 2012. [DOI] [PubMed] [Google Scholar]
- O'Connor KW, Loughlin PJ, Redfern MS, Sparto PJ. Postural adaptations to repeated optic flow stimulation in older adults. Gait Posture 28: 385–391, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler HE. (editor) Attention. Hove, East Sussex, UK: Psychology, 1998, p. viii. [Google Scholar]
- Peterka RJ, Loughlin PJ. Dynamic regulation of sensorimotor integration in human postural control. J Neurophysiol 91: 410–423, 2004. [DOI] [PubMed] [Google Scholar]
- Prado JM, Stoffregen TA, Duarte M. Postural sway during dual tasks in young and elderly adults. Gerontology 53: 274–281, 2007. [DOI] [PubMed] [Google Scholar]
- Rankin JK, Woollacott MH, Shumway-Cook A, Brown LA. Cognitive influence on postural stability: a neuromuscular analysis in young and older adults. J Gerontol A Biol Sci Med Sci 55: M112–M119, 2000. [DOI] [PubMed] [Google Scholar]
- Redfern MS, Jennings JR, Martin C, Furman JM. Attention influences sensory integration for postural control in older adults. Gait Posture 14: 211–216, 2001. [DOI] [PubMed] [Google Scholar]
- Redfern MS, Muller ML, Jennings JR, Furman JM. Attentional dynamics in postural control during perturbations in young and older adults. J Gerontol A Biol Sci Med Sci 57: B298–B303, 2002. [DOI] [PubMed] [Google Scholar]
- Schmidt RA. A schema theory of discrete motor skill learning. Psychol Rev 82: 225–260, 1975. [Google Scholar]
- Shumway-Cook A, Woollacott M. Attentional demands and postural control: the effect of sensory context. J Gerontol A Biol Sci Med Sci 55: M10–M16, 2000. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott M, Kerns KA, Baldwin M. The effects of two types of cognitive tasks on postural stability in older adults with and without a history of falls. J Gerontol A Biol Sci Med Sci 52: M232–M240, 1997. [DOI] [PubMed] [Google Scholar]
- Sienko KH, Balkwill MD, Oddsson LI, Wall C. Effects of multi-directional vibrotactile feedback on vestibular-deficient postural performance during continuous multi-directional support surface perturbations. J Vestib Res 18: 273–285, 2008. [PubMed] [Google Scholar]
- Sienko KH, Balkwill MD, Wall C 3rd. Biofeedback improves postural control recovery from multi-axis discrete perturbations. J Neuroeng Rehabil 9: 53, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienko KH, Vichare VV, Balkwill MD, Wall C 3rd. Assessment of vibrotactile feedback on postural stability during pseudorandom multidirectional platform motion. IEEE Trans Biomed Eng 57: 944–952, 2010. [DOI] [PubMed] [Google Scholar]
- Speers RA, Paloski WH, Kuo AD. Multivariate changes in coordination of postural control following spaceflight. J Biomech 31: 883–889, 1998. [DOI] [PubMed] [Google Scholar]
- Stevens JA, Mack KA, Paulozzi LJ, Ballesteros MF. Self-reported falls and fall-related injuries among persons aged>or=65 years–United States, 2006. J Safety Res 39: 345–349, 2008. [DOI] [PubMed] [Google Scholar]
- Verghese J, Buschke H, Viola L, Katz M, Hall C, Kuslansky G, Lipton R. Validity of divided attention tasks in predicting falls in older individuals: a preliminary study. J Am Geriatr Soc 50: 1572–1576, 2002. [DOI] [PubMed] [Google Scholar]
- Verhoeff LL, Horlings CG, Janssen LJ, Bridenbaugh SA, Allum JH. Effects of biofeedback on trunk sway during dual tasking in the healthy young and elderly. Gait Posture 30: 76–81, 2009. [DOI] [PubMed] [Google Scholar]
- Wall C., 3rd Application of vibrotactile feedback of body motion to improve rehabilitation in individuals with imbalance. J Neurol Phys Ther 34: 98–104, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall C 3rd, Kentala E. Control of sway using vibrotactile feedback of body tilt in patients with moderate and severe postural control deficits. J Vestib Res 15: 313–325, 2005. [PubMed] [Google Scholar]
- Wall C 3rd, Weinberg MS, Schmidt PB, Krebs DE. Balance prosthesis based on micromechanical sensors using vibrotactile feedback of tilt. IEEE Trans Biomed Eng 48: 1153–1161, 2001. [DOI] [PubMed] [Google Scholar]
- Wall C 3rd, Wrisley DM, Statler KD. Vibrotactile tilt feedback improves dynamic gait index: a fall risk indicator in older adults. Gait Posture 30: 16–21, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney SL, Marchetti GF, Schade A, Wrisley DM. The sensitivity and specificity of the Timed “Up & Go” and the Dynamic Gait Index for self-reported falls in persons with vestibular disorders. J Vestib Res 14: 397–409, 2004. [PubMed] [Google Scholar]
- Wilk CM, Gold JM, Bartko JJ, Dickerson F, Fenton WS, Knable M, Randolph C, Buchanan RW. Test-retest stability of the Repeatable Battery for the Assessment of Neuropsychological Status in schizophrenia. Am J Psychiatry 159: 838–844, 2002. [DOI] [PubMed] [Google Scholar]
- Winter DA. Human balance and posture control during stanading and walking. Gait Posture 3: 193–214, 1995. [Google Scholar]
- Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. J Neurophysiol 80: 1211–1221, 1998. [DOI] [PubMed] [Google Scholar]
- Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol 75: 2334–2343, 1996. [DOI] [PubMed] [Google Scholar]
- Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys Ther 84: 906–918, 2004. [PubMed] [Google Scholar]
- Xsens Technologies B.V. MIi: Miniature Attitude and Heading Reference System (Online). Xsens Technologies B.V., Enschede, The Netherlands. http://www.xsens.com/wp-content/uploads/2013/11/mti-leaflet.pdf [2014]. [Google Scholar]