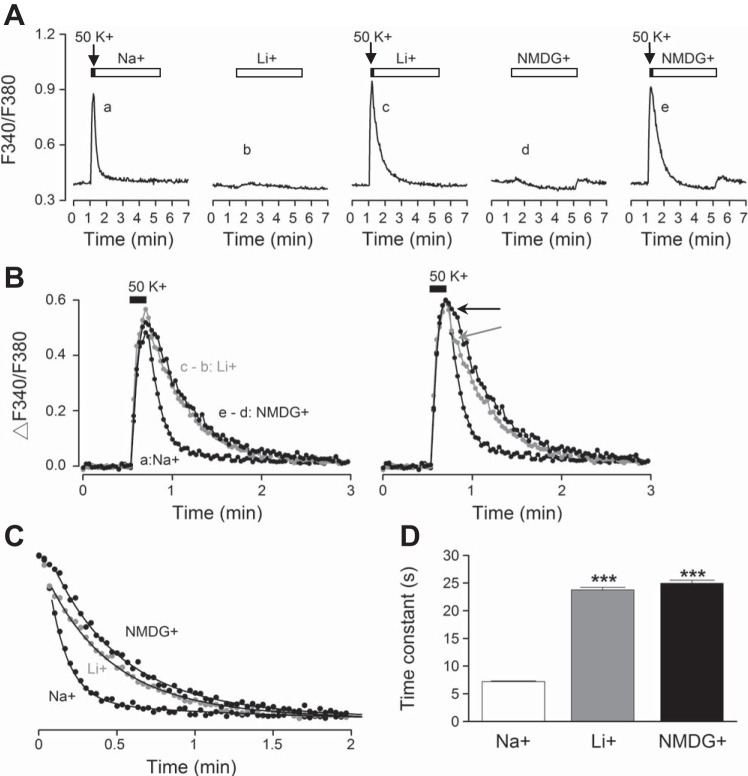
Fig. 5.
Zero Na+ slows the fast decay phase of high-K+-induced Ca2+ transients. A: averaged Ca2+ responses (n = 20 cells) to 50 mM K+ for 10 s followed by return to control solution (140 mM Na+) (a), to 140 mM Li+ for 4 min (b), to 50 mM K+ followed by return to Li+ (c), to 140 mM NMDG+ for 4 min (d), and to 50 mM K+ followed by return to NMDG+ (e). B, left: comparison of the Ca2+ responses that return to Na+ (a), to Li+ (c − b), and to NMDG+ (e − d) indicates a slowing effect of Na+-free solutions on the fast decay phase. Right: normalization of Ca2+ responses in Na+ and Na+-free solution indicates the time when Li+ (marked by gray arrow) and NMDG+ (marked by black arrow) began to slow the Ca2+ decay. C: time courses of Ca2+ decay expanded for curve fitting to obtain decay time constants. The smooth curves through the data points were calculated with a fast time constant of 7.2 s, 24 s, and 25.2 s for Na+, Li+, and NMDG+, respectively. D: statistics showing a 3-fold increase in the fast decay time constants by Na+-free solutions (n = 20 cells). ***P < 0.001. Daytime recordings (ZT 7).