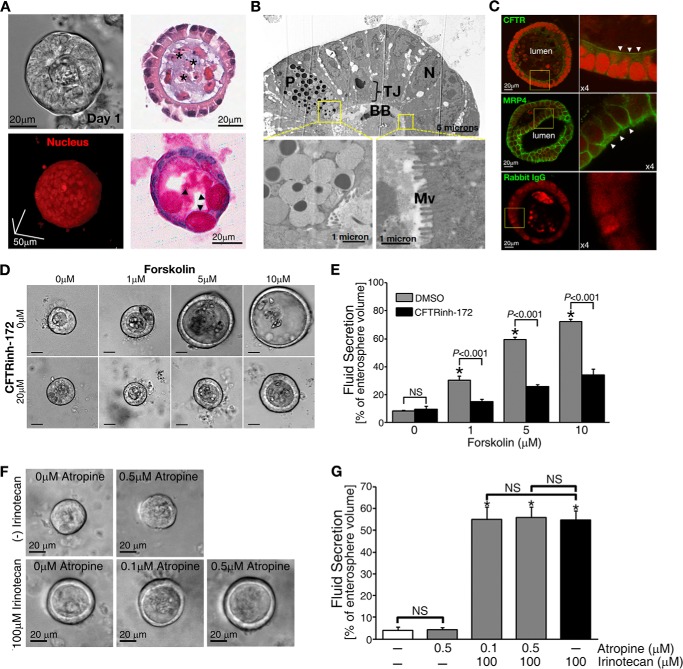
FIGURE 2.
Enterospheres developed from the small intestine of adult mice showed robust CFTR-mediated fluid secretion. A, representative phase-contrast image (upper left) and two-photon micrograph of a three-dimensional cultured enterosphere in Matrigel (lower left). Upper right, histological analysis of enterospheres by HE staining; lower right, periodic acid/Schiff staining. *, luminal area. Arrowheads indicate goblet cells in the enterosphere. B, electron microscope micrographs showing paneth cell (P), brush border (BB), and microvilli (Mv) in the enterosphere (TJ: tight junction; N: nuclei). C, immunohistochemistry staining showing the apical (indicated by arrowheads) localization of CFTR and MRP4 on the enterosphere. Rabbit IgG is used as a negative control. D, forskolin treatment induced CFTR-mediated fluid secretion into the luminal area of the enterospheres in a dose-dependent manner. A specific CFTR channel blocker, CFTRinh-172, inhibited the secretion. Scale bars = 20 μm. E, the data were quantified from experiments as represented in Fig. 1D. F, representative phase-contrast images of fluid secretion in enterospheres from Mrp4+/+ mice in response to irinotecan with or without atropine, an anticholinergic agent. Atropine did not affect irinotecan-increased fluid. G, the data were quantified from experiments as represented in Fig. 1F. All fluid secretion data from enterospheres are presented as the mean ± S.E. (n ≥ 10 enterospheres per group). At least three individual experiments were performed using different mice. *, significant difference as compared with 0 μm irinotecan-treated without atropine group; NS, not significant (p ≥ 0.05).