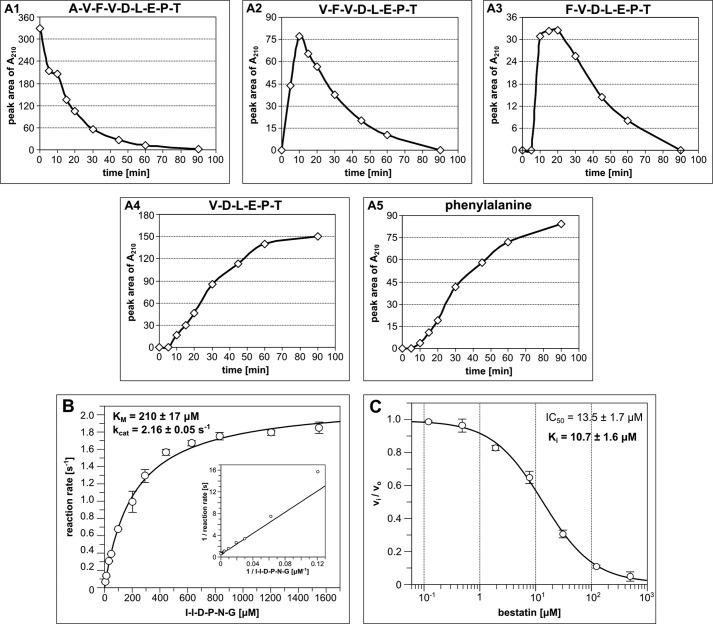
FIGURE 9.
Quantitative analysis of Avi-NaalL aminopeptidase activity. A, time-dependent analysis of the peptide 52 cleavage by Avi-NaalL. Recombinant Avi-NaalL was mixed with peptide 52 in final concentrations of 0.1 and 100 μm, respectively. Reactions were performed at 37 °C and were stopped at times ranging from 5 to 90 min and analyzed by LC/MS. The relative amount of individual peptides or amino acids is plotted against reaction time in A1–A5. B, determination of kinetic parameters of model peptide substrate (I↓IDPNG) cleavage by Avi-NaalL. The enzyme was incubated with various concentrations of substrate, ranging from 6.3 to 1600 μm, for 15 min at 37 °C. The reaction mixtures were subsequently analyzed using HPLC. Each reaction was done in duplicate, and the result is shown as a mean with S.E. (error bar). The kinetic parameters were obtained by non-linear fit of the data using GraFit version 5.0.11 (Erithacus Software Ltd.). The reciprocal linear plot is also illustrated for comparison. C, determination of inhibition constant of aminopeptidase specific inhibitor bestatin toward Avi-NaalL. Various concentrations of bestatin, ranging from 0.1 to 500 μm, were incubated with Avi-NaalL and a model substrate (I↓IDPNG) for 15 min at 37 °C. The reaction mixtures were subsequently analyzed using HPLC. Each reaction was done in duplicate, and the result is shown as a mean with S.E. (error bar). The IC50 value was obtained by non-linear fit of the data using GraFit version 5.0.11 (Erithacus Software Ltd.), and the Ki value was then calculated using the Cheng-Prussoff equation for competitive mode of inhibition (31).