FIGURE 4.
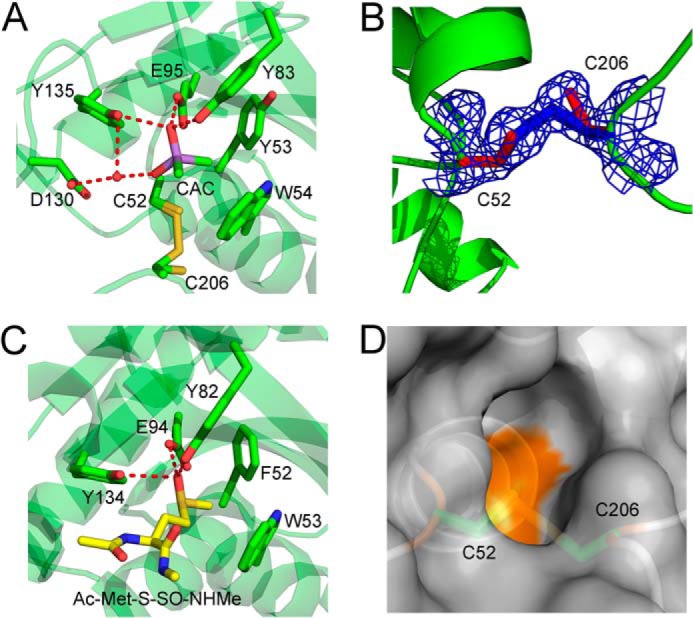
The Cd-MsrA active site shows the first disulfide bond of the relay mechanism and a cacodylate molecule mimicking the substrate. A, the Cd-MsrA active site shows that Cys52 and Cys206 can both be in the reduced form, as in a disulfide bond state. Furthermore, a cacodylate molecule, shown in a purple stick representation, forms hydrogen bonds with Tyr83, Glu95, and Tyr135 and interacts with Asp130 via a water molecule. The two methyl groups of the cacodylate molecule are oriented toward the hydrophobic pocket composed of Tyr53 and Trp54, on the other side of the active site. The Cys52, Tyr53, Trp54, Tyr83, Glu95, Asp130, Tyr135, and Cys206 residues are shown in stick representation. B, the electron density around Cys52 and Cys206 is shown at 1.2σ contour level. C, the N. meningitidis MsrA (Protein Data Bank code 3BQF) active site shows stabilization interactions between the substrate, Ac-Met-S-SO-NHMe (shown in a yellow stick representation), and the conserved hydrogen bond donors Tyr82, Glu94, and Tyr134. These residues, as well as Phe52 and Trp53, are shown in a green stick representation. D, the disulfide bond between Cys52 and Cys206 is not surface-exposed. Cd-MsrA is shown in a gray surface representation, whereas Cys52 and Cys206 are shown in a green stick representation. The figures were generated using MacPyMol (Schroedinger, LLC).
