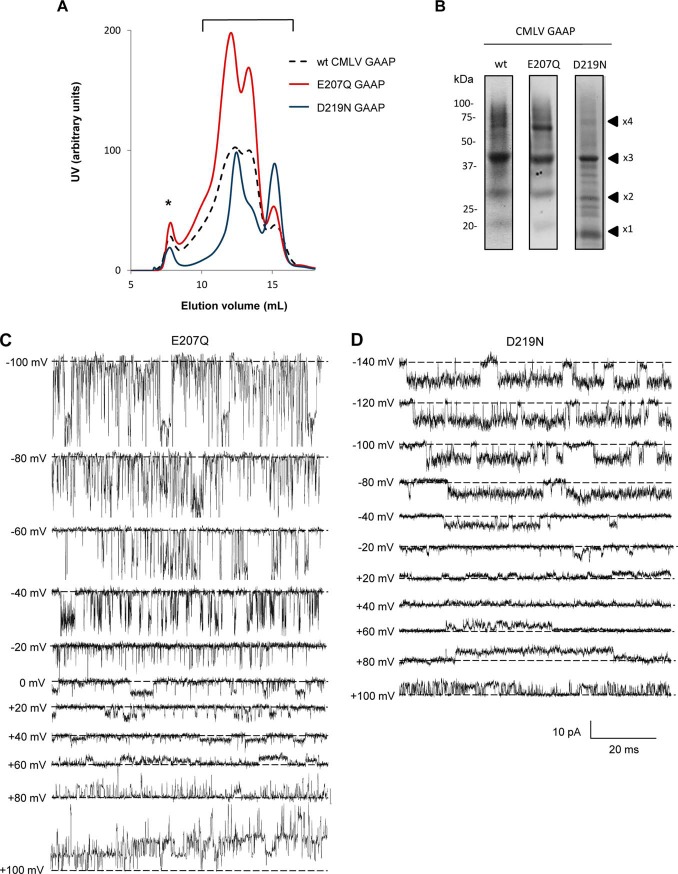
FIGURE 5.
Mutant vGAAPs form functional channels. A, SEC profile of purified CMLV GAAPs. *, protein aggregation peak. Fractions corresponding to the UV peaks of the various monomeric and oligomeric forms of CMLV GAAP (bracket) were pooled and concentrated, and their contents were analyzed (B) by non-reducing SDS-PAGE and Imperial staining. The expected positions of the monomeric (×1) and oligomeric proteins (×2, ×3, and ×4) are shown. C and D, representative traces of spontaneous single-channel openings from vGAAPs with the indicated mutations recorded under asymmetric ionic conditions (see “Experimental Procedures”) at the indicated voltages. The closed state is indicated by the dotted lines.