Abstract
Paroxetine is a selective serotonin reuptake inhibitor (SSRI) that is currently available on the market and is suspected of causing congenital malformations in babies born to mothers who take the drug during the first trimester of pregnancy. We utilized organismal performance assays (OPAs), a novel toxicity assessment method, to assess the safety of paroxetine during pregnancy in a rodent model. OPAs utilize genetically diverse wild mice (Mus musculus) to evaluate competitive performance between experimental and control animals as they compete amongst each other for limited resources in semi-natural enclosures. Performance measures included reproductive success, male competitive ability and survivorship. Paroxetine-exposed males weighed 13% less, had 44% fewer offspring, dominated 53% fewer territories and experienced a 2.5-fold increased trend in mortality, when compared with controls. Paroxetine-exposed females had 65% fewer offspring early in the study, but rebounded at later time points. In cages, paroxetine-exposed breeders took 2.3 times longer to produce their first litter and pups of both sexes experienced reduced weight when compared with controls. Low-dose paroxetine-induced health declines detected in this study were undetected in preclinical trials with dose 2.5-8 times higher than human therapeutic doses. These data indicate that OPAs detect phenotypic adversity and provide unique information that could useful towards safety testing during pharmaceutical development.
Keywords: intraspecific competition, pharmacodynamics, reproductive success, semi-natural enclosures, SSRI, toxicity assessment
1. Introduction
Selective serotonin reuptake inhibitors (SSRIs) are the most commonly prescribed antidepressants and are used to treat the majority of depression cases during pregnancy (Meunier et al. 2013). Paroxetine [Paxil®, GlaxoSmithKline (GSK), Brentford, England] became commercially available in 1992 and has been used to treat patients with depression, anxiety and other mood disorders. Paroxetine, along with other SSRIs, are suspected of causing congenital cardiac defects and pulmonary hypertension when a fetus is exposed during the first trimester; however, these epidemiological studies are controversial where some studies find correlative evidence (Williams and Wooltorton 2005, Bérard et al. 2007, Diav-Citrin et al. 2008, Ellfolk and Malm 2010) and others do not (Kulin et al. 1998, Källén and Otterblad Olausson 2007). Despite this controversy, in 2005, the Food and Drug Administration (FDA) requested that paroxetine be labeled as a class D drug (positive evidence of human fetal risk) and issued a warning that paroxetine exposure in the first trimester may potentially cause birth defects (FDA 2005).
The preclinical assessment of paroxetine followed the typical protocol in which the drug was tested to determine whether it was mutagenic, carcinogenic, teratogenic or if it caused infertility (GSK 2013). No genotoxic effects were detected in rodent cells, and tumors were detected in mice and rats at doses 2-3.9 times the maximum recommended human dose (GSK 2013). Teratogenicity was not assessed in mice and no teratogenic effects were in observed in rats when given a dose > 8 times higher than human therapeutic doses (GSK 2013). Paroxetine effects on fertility were not assessed in mice, but the drug impaired fertility in rats at high doses; that is, when females were given doses 2.5-fold higher and when males were given > 8 times higher than human therapeutic doses (GSK 2013). Another traditional assay that is evaluated during preclinical trials is the functional observational battery (FOB). FOBs consists of several important behavioral assays and autonomic tests to evaluate whether a substance causes neurotoxicity (Moser 2011), however, no data from such tests are reported for paroxetine exposure (GSK 2013). After successful completion of preclinical assessment, paroxetine was deemed safe and continued onto clinical trials until it was released onto the market.
Like paroxetine, many medications once considered safe are found to cause unacceptable health consequences after public release. On average, 73% of pharmaceuticals fail during clinical trials (Lipsky and Sharp 2001) and 10% of FDA approved pharmaceuticals are recalled after market release (Schuster et al. 2005), despite the 12-15 years of research and $1.4 billion average cost associated with each drug during development (Miller 2012). One cause of the high pharmaceutical failure rate is the inability of current toxicity assessment methods to detect cryptic, or otherwise undetectable, adversities during preclinical trials, particularly those present at doses near therapeutic levels and/or those occurring at low incidences.
We have developed a novel toxicity assessment research method that may be useful during preclinical assessment, known as the organismal performance assay (OPA). In several instances, OPAs have proven capable of detecting mammalian health declines that were not visible to standard laboratory methodologies. OPAs utilize genetically diverse wild-derived mice (Mus musculus) that compete amongst each other for limited resources in semi-natural enclosures, which allows for direct competition between treatment and control individuals. OPAs assess the quality of individual mice (organisms) in terms of Darwinian fitness (i.e., reproductive success) and components leading to fitness (i.e., survivorship and male competitive ability), while residing in a naturalistic environment where the stresses that have shaped their evolutionary history are present. The sensitivity of the OPA derives from the fact that wild mice under social competition allows small changes in behavior or physiological performance and otherwise cryptic effects of toxicity to be manifested as measureable negative outcomes; such as relegation to inferior habitat and reduced reproduction and survival. Consequently, any degradation in almost any physiological system caused by a treatment will be detectable by the inability of mice to perform comparable to controls with whom they compete and will be revealed in OPA endpoint measures. OPAs have previously been used to quantify the adverse effects of sibling-level and cousin-level inbreeding (Meagher et al. 2000, Ilmonen et al. 2008), harboring a selfish gene (Carroll et al. 2004) and recently, they were the first assay to reveal the adverse effects of added sugar consumption at human-relevant levels (Ruff et al. 2013). In all of these studies, OPAs found substantial deleterious effects that were missed by current methodologies.
Here OPAs are used to determine if paroxetine exposure near human therapeutic doses during in utero and into early adulthood cause fitness declines in wild mice. If paroxetine exposure adversely affects any physiological system, we hypothesize that exposed individuals will suffer reproduction and survival declines relative to control individuals while competing within enclosures. Furthermore, while generating animals for OPAs, we assessed whether paroxetine exposure negatively affects reproduction of exposed breeders and the weight of the resulting offspring.
2. Materials and Methods
2.1. Animals
Wild-derived outbred house mice were used in this experiment. Unlike many genetically inbred mouse strains, wild mice have behavioral characteristics that allow them to function in natural and semi-natural environments (Nelson et al. 2013). In this experiment, individuals were from the 12th generation of the colony described by Meagher et al. 2000. Genetic diversity of this colony was assessed in the 11th generation and found to be comparable to wild populations (Cunningham et al. 2013). Within enclosures and breeding cages, individuals were provided access to food and water ad libitum and maintained on a 12:12 hour light:dark cycle. All procedures were approved by the University of Utah IACUC.
2.2. Drug exposure
Dosing was achieved by incorporating 7.5 g paroxetine (GSK, molecular formula: C19H20FNO3·HCl) into 50 kg of rodent chow (TD.130006; Harlan Teklad, Madison, WI). Mice consuming an average of 3 g of food per day and weigh 20 g will ingest 0.45 mg per day or 22.5 mg/kg/day. Using a standard metabolic rate conversion factor, this is equivalent to a human dose of 1.82 mg/kg/day, or a daily dose of 109.20 mg, assuming the average human weighs 60 kg (Reagan-Shaw et al. 2008). Given that paroxetine is prescribed in the range of 20 – 60 mg/day (Dunner and Dunbar 1992, GSK 2013), our dose is 1.82-fold higher than human therapeutic doses, yet lower than doses used in previous animal studies (Coleman et al. 1999, Rayburn et al. 2000). Although we did not determine serum levels, one study determined that a paroxetine dose of 30 mg/kg/day achieved serum levels in mice that were comparable to human serum levels when taking the highest therapeutic dose (Coleman et al. 1999).
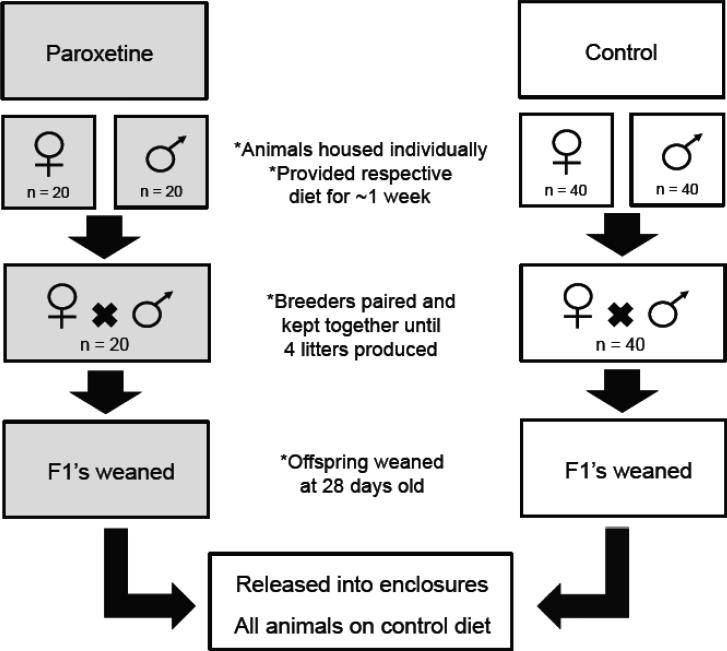
Sixty breeder pairs were selected for this experiment; 20 pairs were exposed to paroxetine while the remainder served as controls. The asymmetry in cage number is due to the production of additional control animals for another study. Prior to breeding, animals were individually housed and provided with their respective diets. To maximize the chances of detecting adverse effects, both females and males were exposed to paroxetine prior to breeding (females were exposed to paroxetine eight days prior and males five days). Exposure to paroxetine continued when breeders were paired. By exposing both females and males, we were consistent with previous rodent studies (Coleman et al. 1999, Rayburn et al. 2000, El-gaafarawi et al. 2005), and it is likely that any the adverse effects detected in the progeny are due to in utero exposure because birth defects have been observed in humans when women are prescribed paroxetine during pregnancy (Diav-Citrin et al. 2008). Breeding pairs were kept together until a maximum of four litters were produced to ensure enough animals for OPA assessment. At 28 days of age, pups were weaned and housed in same-sex sibling cages. Upon weaning, individual weight, sex and litter size data were collected and paroxetine exposure continued until animals were released into semi-natural enclosures (Figure 1). By exposing offspring in utero and through early adulthood (rather than stopping the exposure at weaning), the duration maximized the ability of OPAs to detect health consequences. This is because once animals were released into enclosures they were all fed the control diet, as currently, we are unable to keep animals on their respective diets while they are free ranging during OPAs. Upon release into enclosures, both paroxetine- exposed and control animals were provided with the control diet ad libitum; switching the paroxetine-exposed animals to the control diet (rather than switching control animals to the paroxetine diet) was a more conservative approach of detecting fitness impacts, because OPAs would then be assessing the effects of a previous exposure.
Figure 1. Schematic of paroxetine exposure.
Sixty females and sixty males were selected and individually housed. Twenty females and twenty males were exposed to 22.5 mg/kg/d of paroxetine approximately one week prior to pairing while the remaining individuals received the control diet. Breeders were paired and kept together until a maximum of four litters were produced; exposure to paroxetine continued. Once litters were 28 days old, offspring were weaned and separated into cages with same sex siblings; exposure to paroxetine continued. Upon early adulthood, males and females from both the paroxetine and control treatments were released into semi-natural enclosures. Males were on average 14.3 (SEM ± 0.74) weeks old and females were 18.9 (SEM ± 0.80) weeks old at the time of enclosure release. Once animals were in enclosures, paroxetine exposure ceased and all animals were provided with the control diet. Gray shaded boxes depicts when paroxetine exposure occurred.
2.3. Semi-natural enclosures
Enclosures have previously been described in Ruff et al. 2013. Briefly, the indoor enclosures are approximately 30 m2 and consist of two types of territories, optimal (n = 4) and suboptimal (n = 2). Each optimal territory contained a defendable box with multiple dark nesting sites and direct access to food. Suboptimal territories contained two nesting boxes exposed to light and had indirect access to food. Territories were separated by hardware mesh that is easily climbed, but added an element of spatial complexity (Figure S1).
Five independent OPA populations were established and maintained for 28 weeks. OPA populations consisted of eight males and 14-16 females, for a total of 116 animals (40 males, 76 females); these animals are referred to as population founders. Half of the individuals of each sex were paroxetine-exposed while the remainder served as controls; this population structure allows paroxetine individuals to directly compete with control individuals for mates, resources and territories. Enclosure space and population size created a population density within the range observed in the wild (Sage 1981).
Upon release into enclosures, male mice were on average 14.3 (SEM ± 0.74) weeks old and females were 18.9 (± 0.80) weeks old. Males were released into enclosures with non-experimental females to allow males to establish territories, which prevented incidental breeding, or random mating, during the initial social chaos of population formation. One week later, after males established their social hierarchy, non-experimental females were then removed and replaced with experimental females. Male founders were unrelated at the cousin level or above with the exception that three populations consisted of one set of brothers and one population consisted of two sets of brothers. Additionally, all populations consisted of sister pairs and four populations consisted of sister trios. At most, one male per treatment was in a population containing two of his sisters. When relatedness was present, it was balanced between treatments.
2.4. Reproductive success
Reproductive success of founders was determined by removing and genotyping all offspring in enclosures. Every five weeks, research personnel conducted a “pup sweep,” in which all offspring were removed, sacrificed and had a tissue sample collected for genetic analyses. As the gestation period of mice is three weeks, the first pup sweep occurred on week eight, then, every sweep following occurred on five-week intervals; this sweep schedule prevented offspring from reaching sexual maturity in enclosures. A total of 872 samples were collected with an average of 174.4 ± 17.1 (M ± SEM) offspring per population.
In three of the five populations, reproductive success was determined on a population-level by examining sex-specific allelic variants that have been previously described in Meagher et al. 2000. Briefly, non-overlapping allelic variants were assigned to founders of each treatment (paroxetine-exposed or control). Females were selected upon allelic variants of the mitochondrial genome and males upon the Y-chromosome. The output of male reproductive success in these populations is based solely upon male offspring, as females do not possess Y-chromosomes. Reciprocal markers were assigned across populations to control for confounding effects. Mitochondrial genotypes were assessed in 626 samples (three of five populations) and obtained for 100% of offspring. Y-chromosome genotypes were assessed in all five populations to determine male reproductive output and the sex ratio of offspring. Of the 872 offspring, 414 Y-chromosome genotypes were obtained suggesting that 95% of all males were typed if the sex ratio was 1:1; successfully genotyping nearly all of the progeny allowed for a thorough analysis of founder reproductive success.
To gain a better understanding of individual-level reproductive success, parentage analysis was conducted in two populations using multiple microsatellite loci. Female reproductive success determined by microsatellite loci were converted to population level readouts (i.e., number of pups per treatment rather than number of pups per individual) and combined with the mitochondrial data for analysis. Between six and 17 autosomal microsatellite loci were amplified, scored and analyzed in a stepwise fashion. Loci used were: d1mit251, d1mit449, d3mit22, d3mit312, d3mit333, d4mit205, d5mit139, d6mit138, d9mit232, d9mit251, d12mit277, d14mit128, d17mit24, d17mit62, d17mit82, d17mit102 and d19mit110. Primer sequences were obtained from the Mouse Genome Informatics website, The Jackson Laboratory, Bar Harbor Maine (http://www.informatics.jax.org/ accessed March 2014). Primers were tagged with either CY-5 or CY-3 fluorescent dye. DNA samples were PCR-amplified and then run on 14” x 17”, 6.25% denaturing acrylamide gel at 40 W for three to seven hours (locus dependent). Gels were imaged on a Typhoon Scanner 8600 using ImageQuant software (Amersham Biosciences, Piscataway, NJ).
Parentage via multiple microsatellites was assigned using Cervus 3.0 (Kalinowski et al. 2007). Genotypes of all candidate mothers and fathers and all offspring within each population were used to calculate allele frequencies. Simulations were run 10,000 cycles with an error rate of 1% to derive a delta score. Assigned parents were accepted when the trio confidence of mother, father and offspring was 95%. Using this rule, 91% (187/205) of one population was genotyped and 75% (147/195) of the second population was genotyped.
2.5. Male competitive ability
For identification purposes, all animals were given a unique ear punch and received a passive integrated transponder (PIT) tag (TX1400ST, BioMark, Boise, ID) prior to enclosure release. Two sets of PIT tag antenna and readers (FS2001F-ISO, BioMark, Boise, ID) collected data on OPA populations and were rotated twice weekly among concurrent populations. Antennas were placed on top of feeding stations in each territory of an enclosure. PIT tag data were downloaded to a computer containing data logging software (Minimon, Culver City, CA). Males were assigned as territorial occupants when they possessed > 80% of the reads at a particular location. PIT tag data were collected on female mice, but were not analyzed due to the general lack of information on female dominance behavior.
2.6. Survivorship
Non-invasive health checks were performed daily and extensive enclosure checks every five weeks during pup sweeps. Extensive enclosure checks were limited as to avoid disrupting territoriality that increases the rate of infanticide. When deceased founders were observed, they were removed from the enclosures. The date of death was estimated upon the condition of the corpse. Severely decomposed founders were given a date half way between the date the individual was found and the last date the PIT tag of that individual was recorded.
2.7. Statistical analyses
Cox proportional hazard (PH) models were used for survivorship in enclosures and the time to produce first litter from cage data (JMP 9.0.3, SAS institute Inc., Cary NC). Generalized linear mixed models (GLMM) were used for litter size, reproductive success and male competitive ability (R 3.0.2). Reported SEMs are asymmetric because values have been back-transformed from logarithmic data. Linear mixed models (LMM) were used for wean weight and OPA body weight (R 3.0.2). A complete description of statistical analyses can be found in the supplementary information.
3. Results
3.1. Breeding cage measures
Paroxetine-exposed breeders took 2.3 times longer to produce their first litter when compared to controls (PH; χ2 = 3.98, p < 0.05; Figure 2), however; litter size was not affected by treatment (GLMM; z = −0.58, p = 0.56). Control breeders produced an average of 4.54 (SEM +0.45, −0.41) pups in their first litter, while paroxetine-exposed breeders produced 4.09 (+0.83, −0.75) pups in their first litter. No effect of time (GLMM; z = −0.70, p = 0.49) or time by treatment was detected (GLMM; z = 0.65, p = 0.52). For a complete readout of mixed model results for breeding cage data, see supplementary table 1.
Figure 2. Time until first litter of paroxetine-exposed and control breeders in cages.
In cages, paroxetine-exposed breeders (n = 20) took 2.3 times longer to produce their first litter when compared with control breeders [n = 40, (PH; χ2 = 3.98, p < 0.05)].
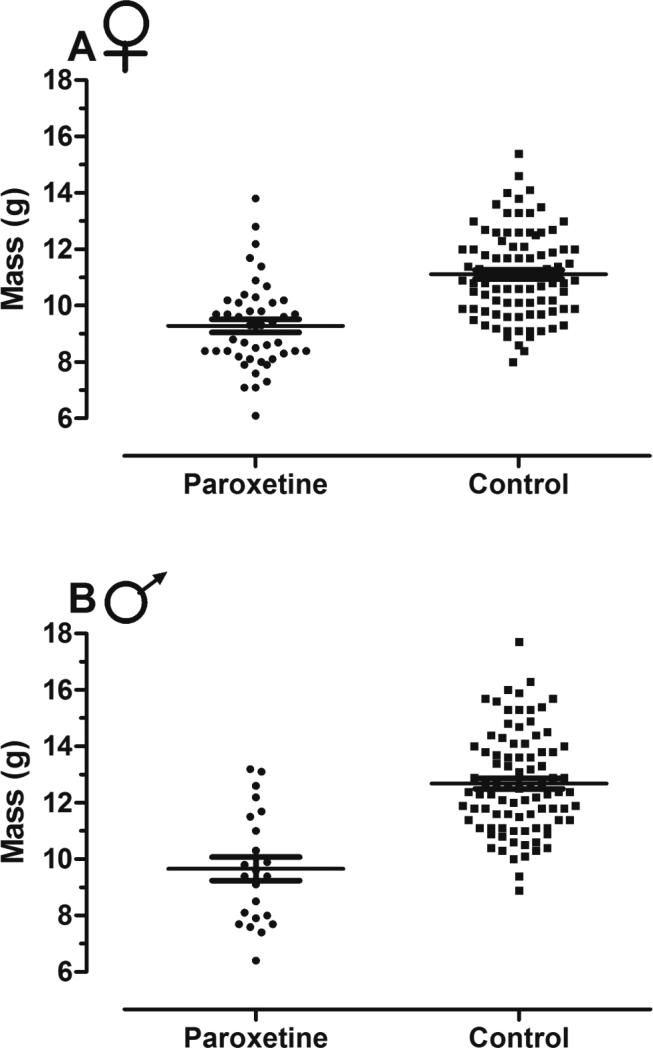
Offspring from paroxetine-exposed breeders weighed less at weaning than offspring from control breeders. Paroxetine-exposed female offspring weighed 16% less than controls with an average of 8.81 g (SEM ± 0.52), whereas control female offspring weighed 10.77 g (± 0.31) (LMM; t = −3.70, p < 0.001; Figure 3A). No effect of parity on weight occurred (LMM; t = 1.80, p = 0.11), nor was there an interaction between treatment and parity (LMM; t = 0.43, p = 0.68). Male offspring from paroxetine-exposed breeders weighed 25% less than controls (LMM; t = −3.83, p < 0.001; Figure 3B), with pups weighing 9.63 g (± 0.73) and control male offspring weighing 12.39 g (± 0.37). No effect of parity (LMM; t = 1.34, p = 0.18) or parity by treatment was detected (LMM; t = 0.41, p = 0.69).
Figure 3. Offspring wean weight from paroxetine-exposed and control breeders in cages.
A) In cages, female offspring from paroxetine-exposed breeders weighed 16% less than controls [n = 24 cages, obs = 134 (LMM; t = −3.70, p < 0.001)]. B) In cages, male offspring from paroxetine-exposed breeders weighed 25% less than male offspring from control breeders [n = 25 cages, obs = 111 (LMM; t = −3.83, p < 0.001)]. Lines represent means and error bars, standard error.
3.2. OPA measures
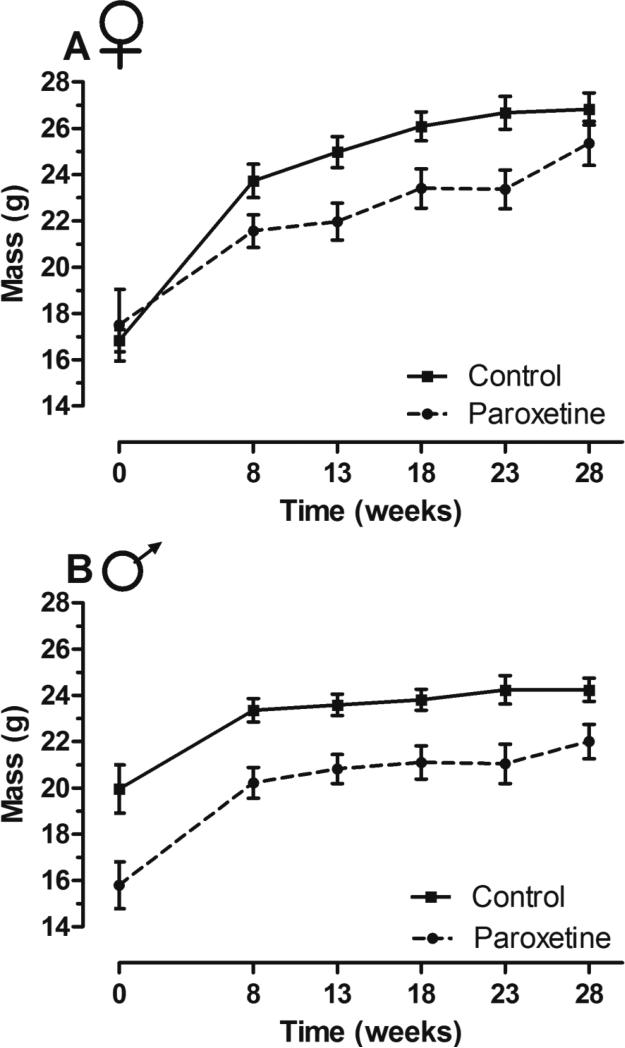
In enclosures, paroxetine exposure had differential affects on body weight between the sexes. Upon enclosure release (model intercept, week zero), no differences were detected in body weight of paroxetine-exposed and control females (LMM; t = −1.04, p = 0.30; Figure 4A). Paroxetine-exposed females weighed on average 19.39 g (SEM ± 1.03) while controls weighed 20.46 g (± 1.25). Both groups of females gained weight over time (LMM; t = 5.12, p < 0.01), due to pregnancy, and no interaction of time by treatment was detected (LMM; t = −1.64, p = 0.11). Paroxetine-exposed males weighed less (LMM; t = −3.94, p < 0.001; Figure 4B) than controls upon enclosure entrance. Paroxetine-exposed males weighed 18.42 g (± 0.87) and controls weighed 21.75 g (± 0.90). Both groups of males gained weight over time (LMM; t = 2.60, p = 0.05), but no time by diet interaction was detected (LMM; t = −0.22, p = 0.83), indicating that paroxetine-exposed males weighed 13% less than controls throughout the duration of the experiment. For a complete readout of mixed model results for OPA body weight measures, see supplementary table 2.
Figure 4. Body weight of paroxetine-exposed and control founders in enclosures.
A) Female body weight did not differ between treatments [n = 76 mice, obs = 386 (LMM; t = −1.04, p = 0.30)]. B) Paroxetine-exposed males weighed 13% less than controls over the duration of the study [n = 40 mice, obs = 179 (LMM; t = −3.94, p < 0.001)]. Lines connect means and error bars represent standard error.
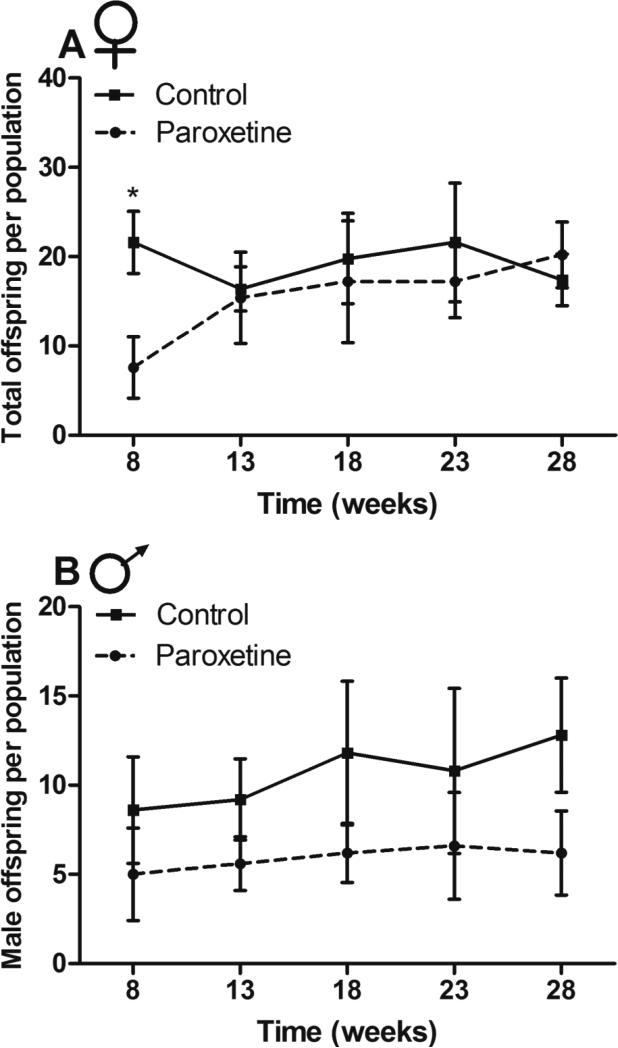
Female reproductive success was hindered by paroxetine exposure (GLMM; z = −5.03, p < 0.0001; Figure 5A) at week eight (model intercept), where mean reproduction of paroxetine-exposed females was 65% less than controls. Paroxetine-exposed females had an average of 10.68 (+1.44, −1.26) offspring per population, while control females had an average of 20.16 (+1.63, −1.51) offspring per population. No effect of time was detected (GLMM; z = −0.63, p = 0.53). However, there was a significant interaction between time and treatment (GLMM; z = 3.95, p < 0.0001), suggesting that paroxetine-exposed female reproduction increased over time. As exposure had significant and opposing effects in regards to the intercept and slope of the linear model, post-hoc t tests were conducted at each pup sweep and the only significant difference detected was at week eight (p < 0.05). For a complete readout of mixed model results for OPA reproduction and competitive ability, see supplementary table 3.
Figure 5. Reproductive success of paroxetine-exposed and control founders in enclosures.
A) Paroxetine-exposed females had 65% fewer offspring at week eight [n = 5 populations, obs = 50 (GLMM; z = −5.03, p < 0.0001)], but had more offspring over time (GLMM; z = 3.95, p < 0.0001). This was confirmed with post-hoc tests indicating that at week eight, reproduction was significantly different; however, no differences were seen at other time points. B) Paroxetine-exposed males had 44% fewer male offspring compared with controls across the study [n = 5 populations, obs = 50 (GLMM; z = −2.72, p < 0.01). Lines connect means of the five populations at each time point for each sex and error bars represent standard error.
Paroxetine exposure also negatively affected male reproductive success as measured by male offspring, where paroxetine exposed males had 44% fewer offspring than controls (GLMM; z = −2.72, p < 0.01; Figure 5B). At week eight (model intercept), mean reproduction of paroxetine-exposed males was on average 5.25 (SEM +1.07, −0.90) male offspring per population per population. Control males sired an average of 8.71 (+1.09, −0.96) male offspring per population. Male founders from both treatments had an increase in reproductive success over time (GLMM; z = 2.16, p = 0.03), but no time by treatment interaction was detected (GLMM; z = −0.51, p = 0.61), indicating that paroxetine-exposed males had reduced reproductive success throughout the study.
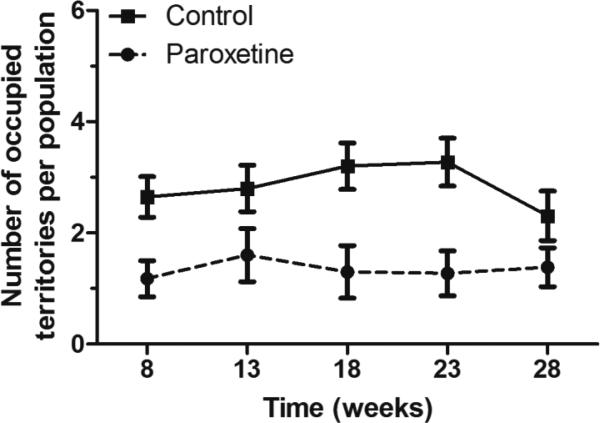
Male competitive ability was adversely impacted by paroxetine-exposure, where paroxetine males occupied 53% fewer territories than controls. At week three (model intercept) control males occupied 47% of the territories, while paroxetine-exposed males occupied 22% territories (GLMM; z = −4.11, p < 0.0001; Figure 6); leaving 31% of the territories undefended. The percent of undefended territories is not unusual because 2/6 (or 33%) were suboptimal and often difficult to defend. There was no difference in territorial occupancy over time (GLMM; z = −0.14, p = 0.89), nor was there a difference in the time by diet interaction (GLMM; z = 0.18, p = 0.86), indicating that the differential acquisition of territories by control and paroxetine-exposed males was consistent across the study.
Figure 6. Competitive ability of paroxetine-exposed and control males in enclosures.
Paroxetine-exposed males occupied 54% fewer territories than controls over the duration of the study [n = 5 populations, obs = 122 (GLMM; z = −4.11, p < 0.0001). Control males occupied 47% of the territories paroxetine-exposed males occupied 22% territories and the remaining 31% of the territories were undefended. A male was considered a territorial occupant if > 80% of his reads were at a particular location. The undefended territories still contain male mice however, < 80% of male reads at this location belonged to a single individual. Points represent the mean number of territories of five populations. To aid in visualization, time points from five-week intervals have been pooled, except for the first time point consisting of 8 weeks. For example, time point week 8 consists of all data points collected from weeks 1-8; time points displayed at week 13 consists of all data points collected from weeks 9-13 and so on. Lines connect means of the five populations and error bars represent standard error.
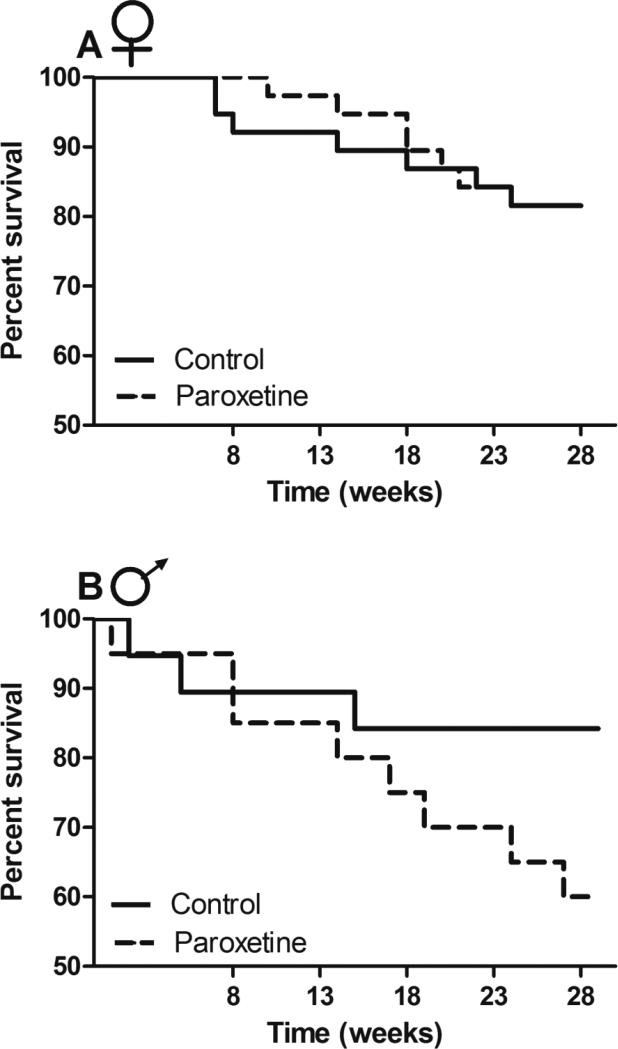
No differences were detected in mortality between paroxetine-exposed and control females (PH; χ2 = 0.66, p = 0.42; Figure 7A). Female mortality rates did not differ in replicate populations (PH; χ 2 = 3.51, p = 0.48), nor was there a difference in the effect of treatment among populations (PH; χ 2 = 3.35, p = 0.50). A marginally significant trend was detected in which male mortality was increased approximately 2.5-fold by paroxetine exposure (PH; χ 2 = 3.27, p = 0.07; Figure 7B). Male mortality rate did not differ in replicate populations (PH; χ 2 = 5.24, p = 0.26), nor was there a difference in the effect of treatment among populations (PH; χ 2 = 3.77; p = 0.44).
Figure 7. Survivorship of paroxetine-exposed and control animals in enclosures.
A) No differences in mortality were detected between treatments in females [n = 76 (PH; χ 2 = 0.66, p = 0.42)] B) Paroxetine-exposed males (n = 40) experienced a marginally significant, 2.5-fold increase in mortality rate when compared to controls (PH; χ2 = 3.27, p = 0.07).
4. Discussion
By utilizing OPAs, we detected numerous adverse effects when animals were exposed to paroxetine in utero through early adulthood. OPA characterization can illustrate phenotypes that are invisible under laboratory conditions but are nonetheless important biologically and knowing that these phenotypes exist allows for the molecular characterization to begin. The phenotypes detected in this study are likely caused by paroxetine disrupting several mechanisms, whose descriptions follow.
In cages, paroxetine-exposed breeders took 2.3 times longer to produce their first litter when compared with controls, potentially caused by endocrine disruption. Within the estrus cycle, a surge of luteinizing hormone (LH) occurs at the time of ovulation allowing for fertilization of the ovum. A reduction in LH levels, along with other important reproductive hormones (i.e., estradiol, progesterone) have been observed after paroxetine exposure in female rats (El-gaafarawi et al. 2005). These hormones play an important role in regulating the function of the female reproductive system and alterations of these hormones may have caused delayed reproduction.
Although litter size did not differ between treatments in cages, paroxetine-exposed females had 65% fewer offspring in enclosures, but at the first pup sweep only. The initial reduction in reproduction may be explained by the endocrine disruptions responsible for the delayed reproduction experienced by females in cages. As the negative effects of paroxetine exposure on reproduction decrease over time, suggests that they are reversible after exposure ceases.
In cages, paroxetine exposure caused reduced wean weight in both sexes. Although litter size has been shown to influence offspring weight (Wurtman and Miller 1976, Epstein 1978), no differences in litter size were detected, suggesting that reduced wean weight is due to paroxetine rather than the confounding effect of litter size. Other rodent studies found similar effects of paroxetine on wean weight (Coleman et al. 1999, Rayburn et al. 2000) and reduced birth weight has been observed in human babies that underwent in utero exposure (Diav-Citrin et al. 2008). Upon enclosure entrance, paroxetine exposure no longer affected female weight, though a trend was observed wherein they gained weight at a decreased rate relative to controls, possibly due to reduced pregnancies. Paroxetine-exposed males however, weighed less at weaning and continued to weigh less throughout the duration of OPA assessment.
Paroxetine-exposed males were less competitive and dominated 53% fewer territories than controls. Decreased body weight likely explains decreased competitive ability, as males must engage in physical competitions to obtain and defend territories. The benefit of a relatively larger body weight on competitive ability is well established to be advantageous in territorial acquisition and defense in mice [e.g. (van Zegeren 1980, Krackow 1993)]. Another possible explanation is altered hormone levels. Testosterone plays a large role in male behavior, especially competitive behaviors in mice (Zielinski and Vandenbergh 1993). El-Gaafarawi et al. (2005) found that when male rats were exposed to therapeutic doses of paroxetine, serum levels of testosterone were significantly reduced and estradiol levels were increased. Though a mechanistic cause is suggested here, the adverse effects on organismal competition have not previously been demonstrated.
Paroxetine-exposed males experienced reduced reproductive success, producing 44% fewer offspring than controls. Previous OPA studies reveal that dominant males sire > 80% of offspring within enclosures (Carroll et al. 2004) and therefore differences in competitive ability likely explain a large portion of the differential reproduction, though other mechanisms are possible. A trend was observed in which paroxetine-exposed males suffered greater mortality and thus even if not significant, it does contribute to the reduced reproduction. Another possibility is that paroxetine has direct negative impacts on the male reproductive system. Paroxetine exposure has been shown to reduce sperm count (Baldwin et al. 1989, El-gaafarawi et al. 2005) and to increase sperm abnormalities in rats when exposed at a human therapeutic dose (El-gaafarawi et al. 2005). Additionally, hormones important for spermatogenesis (Kovacs 2012) have also been altered by paroxetine exposure in rats (El-gaafarawi et al. 2005). These are all potential causes that could lead to reduced reproductive success but additional experiments are needed to decipher the mechanisms causing this phenotype. However, OPAs revealed significant and negative affects on mouse fitness that were missed by conventional methodologies.
An alternative explanation of the observed results in our study is that the animals may have experienced SSRI discontinuation syndrome (i.e., withdrawal). In enclosures, paroxetine-exposed females experienced reproductive impairments that were similar to the impacts detected in cages, suggesting that withdrawal is not occurring; however, there is the possibility that both withdrawal and persistent direct effects of paroxetine could occur concomitantly. Paroxetine-exposed males however, suffered decreased weight, reproduction and competitive ability throughout the duration in enclosures. Withdrawal may explain the male effects; however, no difference in the rate of withdrawal between sexes exists in humans (Black et al. 2000), potentially suggesting no difference between the sexes in mice, though further investigation is needed. Furthermore, as all endpoint measures were affected across the duration of the study it is unlikely that withdrawal explains these effects as one would expect a gradual recovery over time. For example, territoriality is reasserted multiple times daily as dominant males are physically challenged by both subordinate and other dominant males for control and territorial takeovers occur regularly. Therefore, if withdrawal syndrome led to differential initial acquisition of territories it is expected that the advantage of control males to decay over time as the vigor of paroxetine-exposed males returned. To definitively conclude whether the adverse health consequences detected in this study were from persistent effects of paroxetine exposure or withdrawal, additional experiments are needed.
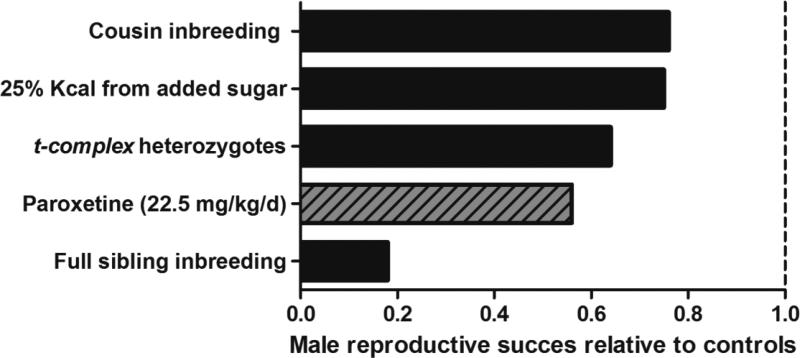
Although fitness declines were detected in both sexes, male mice exposed to paroxetine suffered greater adversity than did females. Differential health consequences between sexes have been detected with the use of OPAs in previous studies. Males have suffered greater negative fitness impacts than females in three previous studies, inbreeding at the cousin level (Ilmonen et al. 2008), inbreeding at the sibling level (Meagher et al. 2000) and when animals were fed a moderate sugar diet (Ruff et al. 2013; Figure 8). Male mice may have been adversely affected by the paroxetine treatment more so than females due to the competitive nature of their social ecology. Since OPA studies generate the same outputs, relative fitness can be compared between treatments and for example, results from this study indicate that paroxetine-exposed male fitness (i.e., reproductive success) is ~25% less than the inbred males born to parents of first cousins (Figure 8).
Figure 8. Comparison of male reproductive success relative to control counterparts from published OPA experiments.
Paroxetine exposure had greater consequences on male fitness (reproductive success) when compared with cousin inbreeding, moderate sugar consumption and a selfish gene, but suffered less fitness consequences when compared to inbreeding at the sibling level (Meagher et al. 2000, Carroll et al. 2004, Ilmonen et al. 2008, Ruff et al. 2013). Figure modified from Ruff et al. 2013.
5. Conclusions
Despite the controversial evidence of paroxetine-induced birth defects in humans, OPAs detected health consequences in mice that underwent paroxetine exposure near human therapeutic doses in utero and into early adulthood. While paroxetine-exposed females recovered from the deleterious effects after the exposure ceased, males continued to experience adverse health consequences throughout their lifetime. Although it is difficult to translate the effects detected in mice to human impacts, we can suggest that if paroxetine is toxic to mice, it likely has similar consequences in humans unless new data arises indicating a species-specific response. These risks should be considered while deciding whether or not to take paroxetine during pregnancy.
There is currently a great need for additional toxicity assessment assays that evaluate toxic substance exposure on an organismal level. The data presented here suggest OPAs can help fulfill this role and may be useful as during pharmaceutical development and if applied, have potential to increase the detection rate of harmful substances during preclinical trials. Likewise, OPAs may also have potential utility in two other divisions of toxicity assessment: 1) screening for possible environmental pollutants before humans and wildlife undergo long-term exposures under the assumption that they are safe and 2) assessing the health consequences of dietary components. However, due to the length of OPA studies, the number of animals used and the quantity of genetic analysis required it seems appropriate that OPAs be applied judiciously to substances that are of key interest such as towards differentiating between two candidate pharmaceuticals being considered for clinical trials, substances that have mixed epidemiological results in regards to human disease (like paroxetine) or substances that are likely to reach high levels in natural environments that may have unknown health consequences. Undeniably, the revelation that low dose paroxetine-exposure negatively affected weight, reproduction, competitive ability and survival is evidence that OPAs have the potential to quantify toxicities that are cryptic in other assays. These organismal phenotypes can now form the basis for investigations to discover the underlying mechanisms.
Supplementary Material
Highlights.
We utilized Organismal Performance Assays (OPAs) to evaluate the safety of paroxetine
OPAs evaluate competition of wild mice living under semi-natural conditions
Wild mice were exposed to a low dose of paroxetine (1.82 times human therapeutic)
Paroxetine-exposed males suffered reduced weight, reproduction and competitive ability
Paroxetine-exposed females experienced reduced reproduction early in the study but rebounded at later time points
Acknowledgments
We thank D. Tripodi for suggesting that we apply OPAs to assess pharmaceuticals; M. Bartlett, S. Eddy, D. Kircher, E. Schwab, M. Sosa, P. Wheatley and C. Young for data collection and genotyping; A. Bwika and J. Gale for animal care. The project was funded by the University of Utah's Technology Commercialization Program and was partially conducted while W. Potts was supported by NSF grant DEB 09-18969 and NIH grant R01-GM109500. S. Gaukler was supported by an NSF GK-12 Educational Outreach Fellowship (DGE 08-41233).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution
S.M.G., J.S.R., G.S.Y. and W.K.P. designed the experiment and obtained funding. S.M.G., T.G., K.A.K., T.K.U., N.M.L., E.L.Y. and L.C.M. maintained enclosures, collected data and genotyped offspring. S.M.G. analyzed the data with the help of J.S.R.. S.M.G. wrote the manuscript. All authors approved and commented upon the manuscript.
References
- Baldwin J, Davidson E, Pritchard A, Ridings J. The reproductive toxicology of paroxetine. Acta Psychiatrica Scandinavica. 1989;80:37–39. doi: 10.1111/j.1600-0447.1989.tb07167.x. DOI: 10.1111/j.1600-0447.1989.tb07167.x. [DOI] [PubMed] [Google Scholar]
- Bérard A, Ramos E, Rey E, Blais L, St-André M, Oraichi D. First trimester exposure to paroxetine and risk of cardiac malformations in infants: the importance of dosage. Birth Defects Research. Part B, Developmental and reproductive toxicolocy. 2007;80:18–27. doi: 10.1002/bdrb.20099. DOI: 10.1002/bdrb.20099. [DOI] [PubMed] [Google Scholar]
- Black K, Shea C, Dursun S, Kutcher S. Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. Journal of Psychiatry and Neuroscience. 2000;25:255–261. [PMC free article] [PubMed] [Google Scholar]
- Carroll LS, Meagher S, Morrison L, Penn DJ, Potts WK. Fitness effects of a selfish gene (the Mus t complex) are revealed in an ecological context. Evolution. 2004;58:1318–1328. doi: 10.1111/j.0014-3820.2004.tb01710.x. DOI: http://dx.doi.org/10.1554/03-544. [DOI] [PubMed] [Google Scholar]
- Coleman FH, Christensen HD, Gonzalez CL, Rayburn WF. Behavioral changes in developing mice after prenatal exposure to paroxetine (Paxil). American journal of obstetrics and gynecology. 1999;181:1166–1171. doi: 10.1016/s0002-9378(99)70102-x. DOI:10.1016/S0002-9378 (99)70102-X. [DOI] [PubMed] [Google Scholar]
- Cunningham CB, Ruff JS, Chase K, Potts WK, Carrier DR. Competitive ability in male house mice (Mus musculus): genetic influences. Behavior genetics. 2013;43:151–160. doi: 10.1007/s10519-012-9577-3. DOI:10.1007/s10519-012-9577-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diav-Citrin O, Shechtman S, Weinbaum D, Wajnberg R, Avgil M, Di Gianantonio E, Clementi M, Weber-Schoendorfer C, Schaefer C, Ornoy A. Paroxetine and fluoxetine in pregnancy: a prospective, multicentre, controlled, observational study. British journal of clinical pharmacology. 2008;66:695–705. doi: 10.1111/j.1365-2125.2008.03261.x. DOI:10.1111/j.1365-2125.2008.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunner DL, Dunbar GC. Optimal dose regimen for paroxetine. Journal of Clinical Psychiatry. 1992;53:21–26. [PubMed] [Google Scholar]
- El-gaafarawi I, Hassan M, Fouad G, El-komey F. Toxic effects of paroxetine on sexual and reproductive functions of rats. The Egyptian Journal of Hospital Medicine. 2005;21:16–32. [Google Scholar]
- Ellfolk M, Malm H. Risks associated with in utero and lactation exposure to selective serotonin reuptake inhibitors (SSRIs). Reproductive toxicology. 2010;30:249–260. doi: 10.1016/j.reprotox.2010.04.015. DOI:10.1016/j.reprotox.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Epstein H. The effect of litter size on weight gain in mice. The Journal of Nutrition. 1978;108:120–123. doi: 10.1093/jn/108.1.120. [DOI] [PubMed] [Google Scholar]
- FDA [March 2014];Public Health Advisory: Paroxetine. 2005 http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm051731.htm http://www.fda.gov.
- GSK Paxil (paroxetine hydrochloride) Tablets and Oral Suspension. Accessed March 2014. 2013 http://www.apotex.com/us/en/products/downloads/pil/paxil_irtb_ins.pdf.
- Ilmonen P, Penn DJ, Damjanovich K, Clarke J, Lamborn D, Morrison L, Ghotbi L, Potts WK. Experimental infection magnifies inbreeding depression in house mice. Journal of evolutionary biology. 2008;21:834–841. doi: 10.1111/j.1420-9101.2008.01510.x. DOI: 10.1111/j.1420-9101.2008.01510.x. [DOI] [PubMed] [Google Scholar]
- Kalinowski ST, Taper ML, Marshall TC. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Molecular ecology. 2007;16:1099–106. doi: 10.1111/j.1365-294X.2007.03089.x. DOI: 10.1111/j.1365-294 X.2007.03089.x. [DOI] [PubMed] [Google Scholar]
- Källén BAJ, Otterblad Olausson P. Maternal use of selective serotonin re uptake inhibitors in early pregnancy and infant congenital malformations. Birth defects research. Part A, Clinical and molecular teratology. 2007;79:301–308. doi: 10.1002/bdra.20327. DOI: 10.1002/bdra.20327. [DOI] [PubMed] [Google Scholar]
- Kovacs W. In: Textbook of Endocrine Physiology. Sixth edition Kovacs W, Ojeda S, editors. Oxford University Press, Inc; New York: 2012. pp. 239–263. [Google Scholar]
- Krackow S. The effect of weaning weight on offspring fitness in wild house mice (Mus musculus domesticus): A preliminary study. Ethology. 1993;95:76–82. DOI: 10.1111/j.1439-0310.1993.tb00458.x. [Google Scholar]
- Kulin N. a, Pastuszak A, Sage SR, Schick-Boschetto B, Spivey G, Feldkamp M, Ormond K, Matsui D, Stein-Schechman a K., Cook L, Brochu J, Rieder M, Koren G. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors: a prospective controlled multicenter study. The journal of the American Medical Association. 1998;279:609–610. doi: 10.1001/jama.279.8.609. DOI:10.1001/jama.279.8.609. [DOI] [PubMed] [Google Scholar]
- Lipsky MS, Sharp LK. From idea to market: the drug approval process. The Journal of the American Board of Family Practice. 2001;14:362–367. [PubMed] [Google Scholar]
- Meagher S, Penn DJ, Potts WK. Male-male competition magnifies inbreeding depression in wild house mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3324–3329. doi: 10.1073/pnas.060284797. DOI: 10.1073/pnas.97.7.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier MR, Bennett IM, Coco AS. Use of antidepressant medication in the United States during pregnancy, 2002-2010. Psychiatric services. 2013;64:1157–1160. doi: 10.1176/appi.ps.201200455. DOI: 10.1176/appi.ps.201200455. [DOI] [PubMed] [Google Scholar]
- Miller H. How is the FDA really doing? Genetic Engineering and Biotechnology News. 2012;32:6–8. [Google Scholar]
- Moser VC. Functional assays for neurotoxicity testing. Toxicologic pathology. 2011;39:36–45. doi: 10.1177/0192623310385255. DOI: 10.1177/0192623310385255. [DOI] [PubMed] [Google Scholar]
- Nelson AC, Cauceglia JW, Merkley SD, Youngson NA, Oler AJ, Nelson RJ, Cairns BR, Whitelaw E, Potts WK. Reintroducing domesticated wild mice to sociality induces adaptive transgenerational effects on MUP expression. Proceedings of the National Academy of Sciences. 2013;110:19848–19853. doi: 10.1073/pnas.1310427110. DOI: 10.1073/pnas.1310427110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayburn WF, Gonzalez CL, Christensen HD, Kupiec TC, Jacobsen JA, Stewart JD. Effect of antenatal exposure to paroxetine (Paxil) on growth and physical maturation of mice offspring. The Journal of Maternal-Fetal Medicine. 2000;9:136–141. doi: 10.1002/(SICI)1520-6661(200003/04)9:2<136::AID-MFM10>3.0.CO;2-Q. DOI: 10.3109/14767050009053439. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. The Federation of American Societies for Experimental Biology Journal. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. DOI: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Ruff JS, Suchy AK, a Hugentobler S, Sosa MM, Schwartz BL, Morrison LC, Gieng SH, Shigenaga MK, Potts WK. Human-relevant levels of added sugar consumption increase female mortality and lower male fitness in mice. Nature communications. 2013;4 doi: 10.1038/ncomms3245. DOI 10:1038/ncomms3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage R. In: The Mouse in Biomedical Research. Foster H, Small J, Fox J, editors. Academic Press; New York: 1981. pp. 40–90. [Google Scholar]
- Schuster D, Lagger C, Langer T. Why drug fail -A study on side effects in new chemical entities. Current Pharmaceutical Design. 2005;11:3545–59. doi: 10.2174/138161205774414510. [DOI] [PubMed] [Google Scholar]
- The Jackson Laboratory . 2013. [March 2014]. http://www.informatics.jax.org. [Google Scholar]
- Williams M, Wooltorton E. Paroxetine (Paxil) and congenital malformations. Canadian Medical Association Journal. 2005;173:1320–1321. doi: 10.1503/cmaj.051421. DOI: 10.1503/cmaj.051421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman J, Miller S. Effect of litter size on weight gain in rats. The Journal of Nutrition. 1976;106:697–701. doi: 10.1093/jn/106.5.697. [DOI] [PubMed] [Google Scholar]
- van Zegeren K. Variation in aggressiveness and the regulation of numbers in house mouse population. Netherlands Journal of Zoology. 1980;30:635–770. [Google Scholar]
- Zielinski WJ, Vandenbergh JG. Testosterone and competitive ability in male house mice, Mus musculus: laboratory and field studies. Animal Behaviour. 1993;45:873–891. DOI: 10.1006/anbe.1993.1108. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.