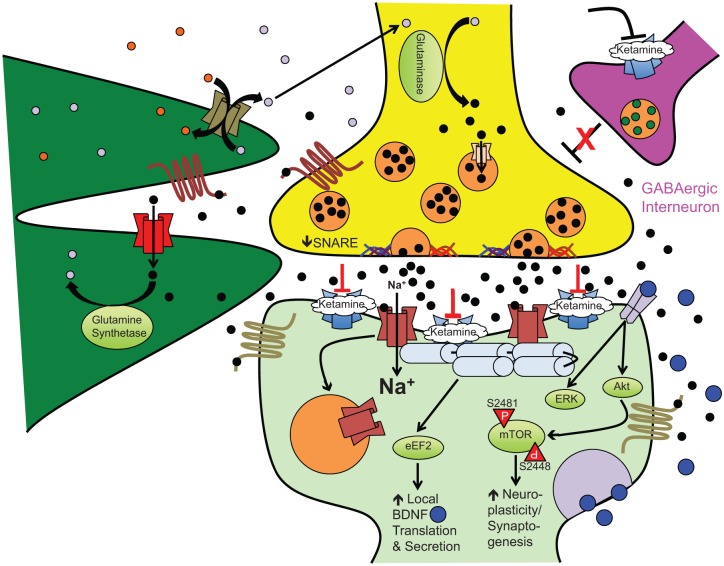
Figure 1.
Ketamine-induced synaptoplasticity and rapid-acting antidepressant efficacy. At subnanesthetic doses, ketamine-induced NMDA receptor antagonism on GABAergic interneurons releases outflow (glutamatergic) neuronal inhibition, for example, cortical pyramidal neurons. This results in an acute glutamate ‘surge’ that then preferentially activates AMPA receptors to increase intracellular sodium (Na+) and fast excitatory currents. This stimulates numerous downstream second messenger/signal transduction cascades leading to the following: (1) eEF2K inhibition, (2) GSK-3 inhibition, and (3) mTOR activation. Other postsynaptic cellular and molecular effects stimulated by ketamine include increased AMPA receptor cycling, increased postsynaptic density protein expression, and dendritic spine morphogenesis (from immature filopodia-shaped spines to mature mushroom-shaped spines). The complex interplay of metabotropic glutamate receptors and exchangers on the surface of neurons and astrocytes regulate synaptic and extrasynaptic glutamate levels to facilitate recycling back to the presynaptic neuron and prevent excitotoxic cell damage/death.
Black circles: glutamate; grey circles: glutamine; blue circles: BDNF; maroon channel: NMDA receptor complex; blue channel: AMPA receptor complex; red channel: glial transporter-1/excitatory amino acid transporter 2 (GLT-1/EAAT2); olive channel: system xC-; peach channel: vesicular glutamate transporter; maroon seven-transmembrane receptor: metabotropic glutamate receptor type 2/3; olive seven-transmembrane receptor: metabotropic glutamate receptor type 1/5; grey dimeric receptor: TrkB receptor. Akt, Ak thymoma/protein kinase B; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; eEF2, eukaryotic elongation factor 2; ERK, extracellular signal-regulated kinase; GABA, γ-aminobutyric acid; mTOR, mammalian target of rapamycin; NMDA, N-methyl-D-aspartate; SNARE, soluble NSF attachment protein receptor (superfamily); TrkB, tropomyosin-related kinase B.