Fig. 3.
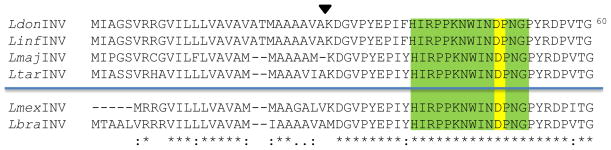
N-terminal end sequence alignment of LdINV and related Leishmania sp. homologs. The N-terminal end-deduced amino acid sequence of the LdINV gene (LdonINV) was compared to those of several Leishmania species present in the TriTryp genome database [L. infantum (LinfINV: LinJ.04.0300), L. major (LmajINV: LmjF.04.0310), L. tarentolae (LtarINV: LtaP04.0290), L. mexicana (LmexINV: LmxM.04.0310), and L. braziliensis (LbraINV: LbrM.04.0350)]. Met1 through Ala28 represents the putative signal peptide of the LdonINV. The arrowhead shown above the LdonINV sequence designates the putative signal peptidase cleavage site of this protein. The KDGVPYE gene-deduced amino acid sequence was verified by N-terminal end AA sequencing of the FPLC-isolated LdINV protein. The area highlighted in green designates the conserved 14 amino acid signature sequence characteristically found in all members of the glyco/hydrolase 32 subfamily of invertases. The yellow-highlighted aa denotes the critical aspartic acid residue within the active/hydrolytic site of this invertase family. The asterisks (*) designate the conservation of amino acid residues among all six Leishmania species; the periods (.) and colons (:) represent amino acids that are semi-conserved substitutions and conserved substitutions, respectively. The blue line denotes geographical separation between the Old World (above) and New World (below) Leishmania species. (Color figure online)
