Abstract
Several nuclear pore-associated factors, including the SUMO-protease Ulp1, have been proposed to prevent the export of intron-containing messenger ribonucleoparticles (mRNPs) in yeast. However, the molecular mechanisms of this nuclear pore-dependent mRNA quality control, including the sumoylated targets of Ulp1, have remained unidentified. Here, we demonstrate that the apparent ‘pre-mRNA leakage’ phenotype arising upon ULP1 inactivation is shared by sumoylation mutants of the THO complex, an early mRNP biogenesis factor. Importantly, we establish that alteration of THO complex activity differentially impairs the expression of intronless and intron-containing reporter genes, rather than triggering bona fide ‘pre-mRNA leakage’. Indeed, we show that the presence of introns within THO target genes attenuates the effect of THO inactivation on their transcription. Epistasis analyses further clarify that different nuclear pore components influence intron-containing gene expression at distinct stages. Ulp1, whose maintenance at nuclear pores depends on the Nup84 complex, impacts on THO-dependent gene expression, whereas the nuclear basket-associated Mlp1/Pml39 proteins prevent pre-mRNA export at a later stage, contributing to mRNA quality control. Our study thus highlights the multiplicity of mechanisms by which nuclear pores contribute to gene expression, and further provides the first evidence that intronic sequences can alleviate early mRNP biogenesis defects.
INTRODUCTION
Export of mature mRNAs out of the nucleus is a pivotal step in the eukaryotic gene expression process and relies on the proper orchestration of several nuclear activities (1). Transcription, processing and assembly of mRNAs into export-competent messenger ribonucleoparticles (mRNPs) are tightly coupled events requiring the timely recruitment of a defined set of factors. Among them, mRNP-associated proteins guide the processing, the localization, and the stability of mRNAs, thus ultimately regulating their cellular fate (2). Acquisition of export competence is associated with the release of the mRNA from the transcription site and the recruitment onto mRNPs of an essential, conserved mRNA export dimer (Mex67-Mtr2 in yeast), which has the unique ability to travel with mRNAs through nuclear pore complexes (NPCs). Several factors have been demonstrated to act at the interface between transcription and NPCs and to facilitate the recruitment of the mRNA export dimer (3): in budding yeast, these include the RNA-binding protein Yra1; shuttling hnRNPs such as Nab2 and Npl3; and Transcription and Export complexes (THO/TREX1 and THSC/TREX2) (1,4). Genetic defects in most of these mRNP components are reported to trigger not only nuclear retention of mRNAs but also quality control (QC) pathways (5).
mRNA QC encompasses nuclear and cytoplasmic mechanisms that prevent the accumulation of improperly processed mRNAs or incompletely packaged mRNPs, which would interfere with protein homeostasis or mRNA biogenesis (6,7). Nuclear QC is mainly achieved by the action of ribonucleases, which, among other targets, catalyze the degradation of faulty mRNPs. The major nuclear RNA degradation activity is carried out by Rrp6 and Rrp44/Dis3, the two catalytic subunits of the multimeric 3′→5′ exonucleolytic nuclear exosome (8). In addition, mRNAs exhibiting abnormal 3′-processing or improper assembly into mRNPs are retained within the nucleus at the vicinity of their transcription site, a process also requiring the catalytic activity of the exosome (9–11). Finally, several NPC-associated proteins have also been proposed to function in mRNA QC prior to export (5,12). While NPC-associated QC has been mainly characterized in the yeast S. cerevisiae, recent reports suggest that it may however be evolutionarily conserved (13–15).
NPC-associated QC has been proposed to rely on the docking of mRNPs at the nuclear basket of NPCs by virtue of their interaction with myosin-like proteins (Mlp) 1 and 2. Physical interactions between a defined set of mRNP components and Mlp1/2, including a direct association of Mlp1 with the hnRNP Nab2, were early identified (16,17) and recently expanded through proteomic analyses (18,19). In addition, overexpression of Mlp1, or its associated cofactor Pml39, leads to the retention of Nab2-containing mRNPs within discrete nuclear foci (20,21). The increased NPC association observed for improperly assembled mRNPs (17), along with the rescue of mRNP assembly mutants upon loss of Mlp1/2 or Pml39 (17,21,22), support the idea that this Mlp1/2 platform may select and release for export properly assembled mRNPs. Ubiquitination-mediated dissociation of Yra1 from mRNPs has been proposed as one of the remodeling mechanisms susceptible to mediate this release (22).
NPC-associated QC also involves the specific nuclear retention of unspliced mRNAs. While the presence of introns in eukaryotic genomes increases their coding capacity, the cellular fate of unspliced mRNAs must be accurately controlled (23). Even in S. cerevisiae, despite representing only 5% of the protein-coding genes, intron-containing genes account for ∼26% of the transcribed mRNAs (24), underlying the importance of pre-mRNA nuclear retention processes. Accordingly, a number of yeast mutants reportedly display a phenotype referred to as ‘pre-mRNA leakage’ (pml), based on the detection of enhanced levels of β-galactosidase expressed from an intron-containing LacZ reporter pre-mRNA. Based on this assay, pre-mRNA retention was shown to require Mlp1 (20) and its partner Pml39 (21); the nucleoporin Nup60 and the nuclear envelope protein Esc1, most likely through their contribution to nuclear basket assembly/integrity (20,25); and the spliceosome-associated factor Pml1 (26). Retained pre-mRNAs are possibly discarded by the action of the endonuclease Swt1, which associates transiently to NPCs (27). How pre-mRNAs are primed for retention versus export is currently unclear; however, the recently reported contribution of the spliceosome–associated SR proteins Gbp2 and Hrb1 to this process positions them as attractive candidates for differentially marking processed transcripts (28).
The same pre-mRNA retention assay also identified a role for the enzyme Ulp1 in NPC-associated QC (25). Ulp1 (SENP2 in mammals) is an NPC-associated SUMO-isopeptidase, which catalyzes both the processing of neosynthesized SUMO prior to conjugation, and the deconjugation of SUMO from dedicated targets, thereby regulating a wide range of cellular processes (29). The sumoylated target(s) of Ulp1 that could contribute to pre-mRNA retention however remained to be identified (5). Of note, we recently reported that Ulp1 regulates the recruitment of the THO complex onto mRNPs through desumoylation of its Hpr1 subunit, contributing to the biogenesis of a subset of stress-inducible mRNPs (19).
In this report, we have investigated the respective contributions of distinct nuclear pore components, including Ulp1, to NPC-associated QC, and searched for their upstream effectors in unspliced mRNA retention. We show that defective sumoylation of the Hpr1 subunit of the THO complex accounts for the apparent ‘pre-mRNA leakage’ phenotype initially scored upon loss of ULP1. Importantly, we demonstrate that altered THO complex activity impinges more severely on the expression of intronless as compared to intron-containing genes, thereby triggering what could be interpreted as ‘pre-mRNA leakage’. Genetic and molecular analyses further reveal that while the THO complex, together with Ulp1 and a subset of nucleoporins, differentially act on intronless and intron-containing genes at the transcriptional level, Mlp1/Pml39 prevent pre-mRNA export at a later stage, highlighting the various mechanisms by which NPCs influence gene expression.
MATERIALS AND METHODS
Yeast strains and plasmids
The strains used in this study are listed in Supplementary Table S1. Yeast cells were grown in standard yeast extract peptone dextrose (YPD) or synthetic complete (SC) media lacking appropriate amino acids. Unless indicated, phenotypes were analyzed following growth at 30°C. ulp1 and mex67–5 strains were grown at 25°C prior to a 2 h shift at 30°C or a 30 min shift at 37°C, respectively. For GAL1 promoter induction, 2% galactose was added for 2 h to cells grown in glycerol-lactate (0.17% YNB, 0.5% ammonium sulfate, 0.05% glucose, 2% lactate and 2% glycerol) supplemented with the required nutrients. For experiments involving tho mutants, galactose induction was performed for 5 h. When indicated, mycophenolic acid (MPA, 100 μg/mL, Sigma) was added to the medium. Construction of plasmids (listed in Supplementary Table S2) was performed using standard molecular cloning techniques.
Gene expression analyses and chromatin immunoprecipitation
Cells carrying LacZ reporters were lysed and assayed for β-galactosidase activity using the NovaBright™ β-galactosidase Chemiluminescent Detection System (Invitrogen) according to the manufacturer's instructions. This assay is 104 more sensitive than the classical colorimetric assay and its dynamic range exceeds five orders of magnitude (30). Relative Light Units (RLU) were normalized to the amount of cells as determined by measurement of the OD at 600 nm.
Total RNAs were extracted from yeast cultures using Nucleospin RNA II (Macherey Nagel) and reverse-transcribed with AMV reverse transcriptase (Finnzymes). cDNA quantification was achieved through quantitative real-time polymerase chain reaction (PCR) with a LightCycler 480 system (Roche) according to the manufacturer's instructions. Sequences of qPCR primers used in this study are listed in Supplementary Table S3. Normalizations were performed using RNA standards (25S rRNA or ACT1) harboring raw expression levels in a similar range as compared to the mRNA of interest.
Chromatin immunoprecipitation was performed as reported (19) except that anti-RNAP II largest subunit antibodies (8WG16, Covance) were added to chromatin extracts supplemented with 0.5% BSA and 50 μg/mL salmon sperm DNA prior to immunoprecipitation. Input and immunoprecipitated DNA amounts were further quantified by real-time PCR as above.
Measurement of 2μ-plasmid levels
2μ-plasmids were recovered from yeast cultures using the QIAprep Spin Miniprep Kit (Qiagen) according to the manufacturer's instructions except that a bead-beating step was introduced to break the cells. The purified DNA was further quantified by real-time PCR as described above.
Cell imaging
Wide-field fluorescence images were acquired using a DM6000B Leica microscope with a 100×, NA 1.4 (HCX Plan-Apo) oil immersion objective and a CCD camera (CoolSNAP HQ; Photometrics). Images were scaled equivalently using MetaMorph 5 (Universal Imaging) and processed with Photoshop CS2 9.0 software (Adobe).
mRNP purification and western blot analysis
mRNP isolation from yeast cells expressing a tagged-version of the nuclear cap-binding complex (Cbc2-protA) was performed as previously described (19). Total protein extraction from yeast cells was performed by the NaOH-TCA lysis method (31). Samples were separated on 4–12% SDS-PAGE gels (Invitrogen) and transferred to PVDF membranes. Western-blot analysis was performed using the following antibodies: monoclonal anti-GFP (1:500, Roche Diagnostics); monoclonal anti-Dpm1 (1:2000, Invitrogen); polyclonal anti-Hpr1 (1:1000) (32); polyclonal anti-Mex67 (1:1000) (32) and polyclonal IgG-HRP (1:5000, Dakocytomation, to detect protA-tagged proteins). Polyclonal anti-SUMO antisera (1:2000) were obtained from Agrobio following immunization of rabbits with recombinant yeast SUMO expressed in bacteria from pET15-HisScSMT3 (see Supplementary Table S2). Quantification of signals was performed based on serial dilutions of reference samples using the ImageJ software.
Statistical analyses
Data are mean ± SD from at least three measurements arising from independent cultures. Asterisks indicate statistical significance (p-values were calculated using two-tailed t-tests assuming unequal variances; [*] p < 0.05; [**] p < 0.01; [ns] not significant).
RESULTS
Ulp1 prevents the ‘pre-mRNA leakage’ phenotype through THO complex sumoylation
To determine which mRNP-associated factor(s) could participate in NPC-associated QC, we systematically assayed pre-mRNA leakage in representative yeast mutants of the mRNA export machinery. For this purpose, we quantified β-galactosidase activities derived from the ‘leakage’ reporter, that contains a synthetic intron (RP51A*) maintaining the frame between the ATG and the LacZ coding sequence, thereby allowing the measurement of pre-mRNA export (Figure 1A). As previously described (20,21,25), these values were normalized to the ones obtained from a similar but intronless LacZ construct (Figure 1A, Supplementary Figure S1A,B). Of note, LacZ expression defects were previously recorded in several mutants impaired in mRNA biogenesis and export (33). We therefore took advantage of a chemiluminescent β-galactosidase assay (30) to accurately monitor pre-mRNA leakage in such mutants; this detection system indeed exhibits an improved sensitivity and linearity over a wide range of enzyme concentrations as compared to the classical colorimetric assay (see Material and Methods). This analysis revealed that the mlp1Δ mutant exhibits a significantly increased ‘leakage’ ratio relative to wt cells (Figure 1B and Supplementary Table S4), in agreement with previous reports (20,21). In contrast, we did not detect any significant increase in pre-mRNA leakage in a mutant of the mRNA export receptor Mex67 (mex67–5) (34) or in mutants of its adaptors Npl3 (npl3Δ), Yra1 (yra1-KR, a mutant impairing Tom1-mediated dissociation of Yra1 from mRNPs) (22) or Nab2 (nab2-F73D, a mutant impairing Nab2 association with Mlp1) (35) (Figure 1B and Supplementary Table S4). Pre-mRNA leakage was also not affected upon loss of Sus1 or Sem1, two subunits of the TREX2 complex (36,37)(Figure 1B and Supplementary Table S4). In contrast, significant levels of apparent ‘pre-mRNA leakage’ were detected in mutants of three of the four subunits of the tetrameric THO complex (Hpr1, Mft1 and Thp2; Figure 1B and Supplementary Table S4). Of note, the fourth subunit of the THO complex, Tho2, could not be assayed due to undetectable LacZ expression from the ‘leakage’ reporter in tho2Δ cells. This observation is consistent with previous studies revealing that Tho2 is the THO complex subunit for which inactivation triggers the most drastic growth and gene expression defects (9,38,39). Our results thus position the THO complex as a possible mediator of NPC-dependent pre-mRNA retention.
Figure 1.
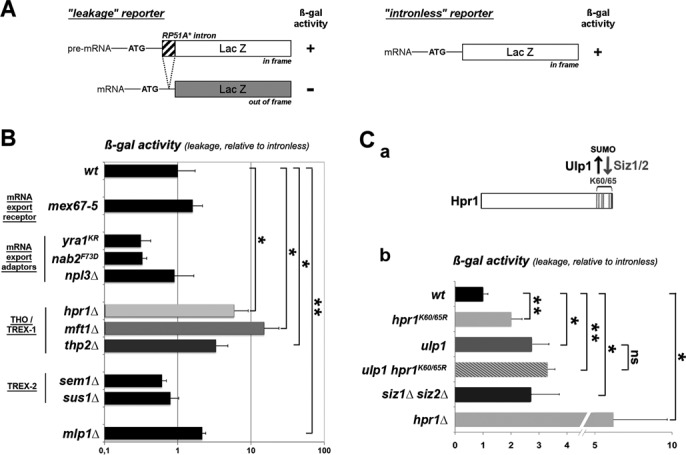
Ulp1 prevents the ‘pre-mRNA leakage’ phenotype through sumoylation of the THO complex. (A) Schematic representation of the LacZ reporters used in this figure. Both ‘leakage’ and ‘intronless’ reporters are galactose-inducible LacZ genes. The ‘leakage’ construct bears a small synthetic intron downstream of the initiation codon, allowing the pre-mRNA to encode β-galactosidase (see Supplementary Table S2). (B) β-galactosidase (β-gal) activities (mean ± SD; n = 4) from the ‘leakage’ reporter were quantified using a chemiluminescent assay as described under Material and Methods and normalized to the values obtained from the intronless construct for the indicated mutants. Values were set to 1 for isogenic wt cells grown under the same conditions. (C) a: the SUMO-ligases (Siz1/2) and protease (Ulp1) target Hpr1 on its C-terminal domain (lysines 60–65, mutated in the hpr1-K60–65R strain) (19). b: β-gal activities (mean ± SD; n = 4) from the ‘leakage’ reporter were normalized to the values obtained from the intronless construct for the indicated mutants. Raw data are provided in Supplementary Table S4.
We recently reported that Ulp1 regulates THO complex recruitment onto mRNPs through sumoylation of its Hpr1 subunit (19). We therefore determined whether Ulp1 could control pre-mRNA retention through Hpr1 sumoylation. For this purpose, we analyzed pre-mRNA leakage in different mutants affecting Hpr1 sumoylation without interfering with its steady-state protein levels (19): (i) mutant cells expressing a non-sumoylatable version of Hpr1 (hpr1-K60–65R), (ii) the double mutant of the Siz1 and Siz2 SUMO-ligases that prevents Hpr1 sumoylation and (iii) a thermosensitive mutant of ULP1 (ulp1–333, thereafter referred to as ulp1) that accumulates sumoylated Hpr1 (Figure 1Ca). Interfering with Hpr1 sumoylation by any of these three ways similarly triggered a ‘pre-mRNA leakage’ phenotype (Figure 1Cb). Of note, we previously reported that mutants leading to either hyper- or hypo-sumoylation of Hpr1 similarly decrease Hpr1 loading onto mRNAs (19). These data thus indicate that defective recruitment of Hpr1 onto the LacZ transcript in Hpr1 sumoylation mutants triggers this ‘pre-mRNA leakage’ phenotype.
Finally, combination of the ulp1 and hpr1-K60–65R mutants did not lead to a synergic phenotype (Figure 1Cb), suggesting that Ulp1 and Hpr1 sumoylation function in a common pathway preventing ‘pre-mRNA leakage’. Taken together, these results thus suggest that the NPC-associated SUMO protease Ulp1 prevents the ‘pre-mRNA leakage’ phenotype through sumoylation of the THO complex.
Differential requirement of the THO complex for expression of intronless and intron-containing LacZ reporters
Our data support the fact that Ulp1 and the THO complex function in a common pathway to prevent the ‘pre-mRNA leakage’ phenotype. However, the THO complex being critical for LacZ mRNA transcription (40), we further examined in detail the two THO complex inactivation mutants (hpr1Δ and mft1Δ), which exhibit the strongest apparent leakage phenotype with our reporter system (Figure 1B). As anticipated, THO complex inactivation dramatically decreased expression of both intronless and ‘leakage’ LacZ constructs at the level of β-galactosidase activities (Figure 2B, top and mid panel). Strikingly however, β-galactosidase activities from the intron-containing ‘leakage’ reporter were less affected as compared to the intronless LacZ reporter (Figure 2B, top and mid panel; compare for example the 1865-fold decrease in β-gal activity from the intronless reporter as opposed to the 211-fold reduction from the ‘leakage’ reporter in the hpr1Δ mutant). Consequently, THO mutants exhibited an apparent increase in relative β-galactosidase activities when normalized to the ones obtained from the intronless reporter (Figure 2C). This raised the possibility that apparent ‘pre-mRNA leakage’ in THO complex mutants actually results from a differential reduction of intronless versus intron-containing LacZ reporter expression.
Figure 2.
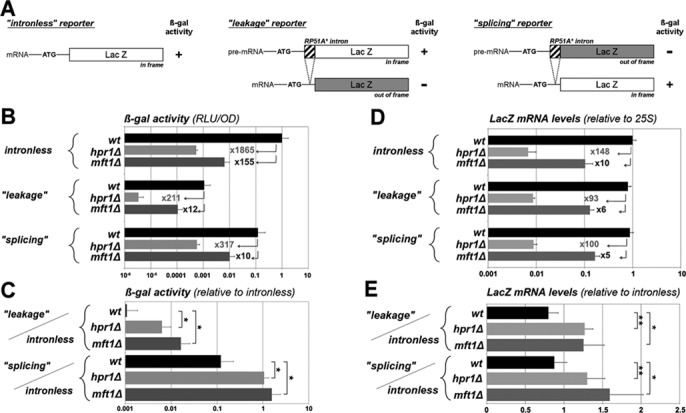
Differential requirement of the THO complex for expression of intronless and intron-containing reporters. (A) Schematic representation of the LacZ reporters used in this figure. Upon expression of the ‘splicing’ reporter, only the spliced mRNA encodes ß-galactosidase. (B) Expression of the three LacZ reporter genes (intronless, ‘leakage’ and ‘splicing’) was monitored by measurement of β-gal activities (RLU/OD, see Material and Methods; mean ± SD, n = 3) in the indicated strains. Values were set to 1 for wt cells carrying the intronless reporter. Fold decreases in tho mutants relative to wt are indicated by numbers. (C) β-gal activities (mean ± SD; n = 3) obtained in panel (B) from intron-containing reporters were normalized to the values obtained from the intronless reporters in the same mutants. Increased relative β-gal activities of ‘leakage’ over intronless reporter were previously used to evaluate the pre-mRNA leakage phenotype, while a decreased ratio of ‘splicing’ over intronless reporters would reflect a splicing defect. (D) Expression of the different reporter genes was monitored by quantifying LacZ mRNA levels by RT-qPCR (normalized to 25S rRNA values; mean ± SD; n = 3) upon extraction of total mRNAs from the indicated strains. LacZ mRNA was detected using LacZ-3′ primers (see Supplementary Table S3); similar results were obtained with LacZ-5′ primers (our unpublished data). Values were set to 1 for wt cells carrying the intronless reporter. Fold decreases in tho mutants relative to wt are indicated by numbers. Note that similar data were obtained when normalizing LacZ mRNA levels to the ACT1 standard (our unpublished results). (E) mRNA levels (mean ± SD; n = 3) obtained in panel (D) from the two intron-containing reporters (‘leakage’ and ‘splicing’ reporters) were normalized to the values obtained from the intronless reporter in the same mutants.
To confirm this hypothesis, the same analysis was performed using a distinct intron-containing LacZ reporter, previously used to evaluate splicing efficiency, in which the same synthetic intron disrupts the frame between the ATG and the LacZ coding sequence (Figure 2A) (20,21,25). With this ‘splicing’ reporter, export and translation of the properly spliced mRNA, but not of the pre-mRNA, account for detectable β-galactosidase activities. The expression of this intron-containing reporter was again less affected as compared to the intronless LacZ gene in tho mutants (Figure 2B, bottom panel). As a consequence, tho mutants displayed enhanced expression levels of this distinct intron-containing reporter relative to the intronless gene (Figure 2C). This demonstrates that inactivation of the THO complex differentially impairs the expression of intronless and intron-containing LacZ genes, regardless of the frame of the intron in respect to the LacZ coding sequence. This further reveals that in THO complex mutants, the ‘pre-mRNA leakage’ phenotype is caused by a differential reduction in LacZ reporter expression, and not by a lack of nuclear retention of intron-containing mRNAs.
We next asked whether this intron-dependent effect of tho mutants on LacZ expression was also observed at the mRNA level. For this purpose, expression of the same LacZ reporters was analyzed by RT-qPCR in hpr1Δ and mft1Δ cells. As expected, THO complex inactivation led to a strong decrease in the detectable amounts of total (e.g. nuclear + cytoplasmic) transcripts from the three reporters (Figure 2D). However, mRNA levels from the two intron-containing reporters were less affected upon THO inactivation as compared to the intronless reporter (Figure 2D). Consequently, THO complex mutants exhibited an apparent increase in relative mRNA levels from intron-containing reporters when normalized to the ones obtained from the intronless reporter, regardless of the frame of the intron with the β-galactosidase coding sequence (Figure 2E). tho mutants therefore exhibit a differential reduction in LacZ mRNA expression depending on the presence of an intron. Importantly, this phenotype does not reflect a differential response of these intronless and intron-containing reporters to any source of transcriptional inhibition; indeed, treatment of cells with a transcription elongation inhibitor (mycophenolic acid, MPA) impaired expression of both intronless and intron-containing reporters to the same extent, as opposed to THO inactivation (Supplementary Figure S2A,B). Likewise, this differential effect of tho mutants on intronless and intron-containing gene expression was not related to the reported function of the THO complex in plasmid maintenance (41); indeed, intronless and intron-containing reporter 2μ-plasmids were checked to be similarly affected in tho mutants (Supplementary Figure S2C). Finally, similar results were observed when the intronless and intron-containing reporters were expressed from centromeric plasmids (Supplementary Figure S3).
Taken together, our results thus show that the apparent ‘pre-mRNA leakage’ phenotype scored in THO inactivation mutants actually reflects LacZ mRNA expression defects that are alleviated in the presence of an intron, regardless of its frame with the LacZ coding sequence (e.g. both with ‘leakage’ and ‘splicing’ reporters, Supplementary Figure S1C).
Intronic sequences can alleviate the transcriptional defects caused by THO complex inactivation
We next wondered whether the ability of an intron to alleviate the LacZ expression defect arising in tho mutants was also shared by natural introns. For this purpose, the poorly spliced (42), synthetic RP51A* intron present in the ‘splicing’ LacZ reporter construct was replaced by distinct natural introns from the PRE3, ACT1 or RPL35A genes (Figure 3A). These introns were previously inserted within heterologous sequences without compromising their splicing or impairing the expression of the constructs (43,44). Consistently, in wt cells, β-gal activities were less severely impacted by the presence of these three introns in the reporter than by the RP51A* intron (Figure 3B). Interestingly, these novel intron-containing constructs were less affected for their expression than their intronless counterpart upon loss of the THO complex (Figure 3B,C; compare in the hpr1Δ mutant, the 468-fold decrease in β-gal activity for the intronless construct as opposed to a maximal 289-fold reduction for the intron-containing constructs). In some cases, the presence of the intron even improved LacZ expression in tho mutants (see for example the RPL35A intron; Figure 3B,C). Distinct natural introns can therefore alleviate the LacZ expression defects caused by loss of the THO complex, albeit to a variable extent depending on the considered intron.
Figure 3.
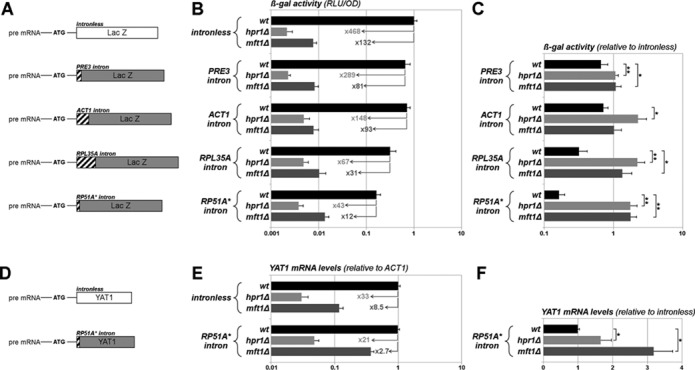
Different introns alleviate the expression defects caused by THO complex inactivation. (A) Schematic representation of the LacZ constructs used in panels (B) and (C). LacZ-based reporter genes bear either the small synthetic intron used previously (RP51A* intron), or natural introns (PRE3, ACT1 and RPL35A introns) downstream of the initiation codon. As for the ‘splicing’ reporter, only the spliced mRNA encodes ß-galactosidase (see Supplementary Table S2, pRS426 constructs). (B) Expression of the LacZ reporter genes was monitored by measurement of β-gal activities (RLU/OD, see Material and Methods; mean ± SD; n = 4) in the indicated strains. Values were set to 1 for wt cells with the intronless reporter. Fold decreases in THO mutants relative to wt are indicated by numbers. (C) β-gal activities (mean ± SD; n = 4) obtained in panel (B) from intron-containing reporters were normalized to the values obtained from the intronless reporter in the same mutants. (D) Schematic representation of the YAT1 constructs used in panels (E) and (F). The natural YAT1 gene was expressed under the control of a galactose-inducible promoter as previously reported (40). The small synthetic RP51A* intron was introduced downstream of the initiation codon (see Supplementary Table S2). (E) Expression of YAT1 reporters was monitored by quantifying YAT1 mRNA levels by RT-qPCR (normalized to ACT1 mRNA values; mean ± SD; n = 4) upon extraction of total mRNAs from the indicated strains. YAT1 mRNA was detected using YAT1–3′ primers (see Supplementary Table S3); similar results were obtained with YAT1–5′ primers (our unpublished data). Values were set to 1 for wt cells carrying the intronless reporter. Fold decreases in tho mutants relative to wt are indicated by numbers. Note that similar data were obtained when normalizing YAT1 mRNA levels to the 25S rRNA standard (our unpublished results). (F) mRNA levels (mean ± SD; n = 4) obtained in panel (E) from the intron-containing construct were normalized to the values obtained from the intronless construct in the same mutants.
We then examined whether the presence of an intron could similarly attenuate the expression defects of an endogenous THO target gene. For this purpose, we replaced LacZ in the intronless and RP51A* intron-containing constructs by YAT1, a S. cerevisiae gene reported to require the THO complex for its expression (40)(Figure 3D). In agreement with this previous study, mRNA levels from the YAT1 intronless construct were strongly decreased in hpr1Δ and mft1Δ cells (Figure 3E). Strikingly, whereas the presence of the intron did not affect YAT1 mRNA levels in wt cells, it significantly rescued their decreased levels in both hpr1Δ and mft1Δ mutants (Figure 3E,F). The presence of an intron can therefore alleviate the expression defect of a natural THO target gene in THO complex mutants.
We finally asked whether the presence of the intron rescues the decrease in LacZ and YAT1 mRNAs by modifying their transcription or their stability in tho mutants. Inactivation of the THO complex was previously reported to reduce mRNA expression by decreasing transcriptional elongation for different model genes (41,45–47), without any detectable changes in mRNA stability in the case of LacZ (48). We thus performed chromatin immunoprecipitation (ChIP) along LacZ and YAT1 genes using an anti-RNA polymerase II (RNAP II) antibody, a method classically used to determine RNAP II recruitment or processivity (45). ChIP analysis of the intronless LacZ gene revealed marked RNAP II occupancy defects in tho mutants (Figure 4A). Both hpr1Δ and mft1Δ mutants exhibited a decreased processivity of the transcription machinery, in agreement with published ChIP data (47) (Figure 4A; note the 50% decrease in the levels of RNAP II associated with the 3′ end of the gene as compared to the 5′ region in the mutants). In addition, the hpr1Δ mutant also exhibited a decreased RNAP II occupancy at the 5′ position (Figure 4A), consistent with previous run-on analyses which had shown that RNAP II pauses from the very first 170bp of LacZ (41). Remarkably, while this latter defect was still observed in hpr1Δ cells in the presence of the intron, the RNAP II processivity defect was no longer detectable along the intron-containing LacZ gene in both hpr1Δ and mft1Δ mutants (Figure 4A; note the similar levels of RNAP II associated with both 5′ and 3′ end of the gene in the mutants). In the case of the intronless YAT1 gene, ChIP analysis revealed a drop in RNAP II occupancy all along the gene in both hpr1Δ and mft1Δ cells (Figure 4B), providing a rationale for the previously reported decrease in YAT1 mRNA levels in these mutants (40)(Figure 3E). Strikingly, while insertion of the intron in the YAT1 gene did not disturb RNAP II recruitment in wt cells, it significantly enhanced RNAP II occupancy all along the gene in both tho mutants (Figure 4B). This positive effect of the intron on RNAP II occupancy along the YAT1 gene well mirrored its impact on YAT1 mRNA amounts in both mutants (Figure 3E). For both YAT1 and LacZ genes, the presence of an intron thus alleviates some of the effects of THO complex inactivation on RNAP II occupancy, thereby explaining the differential requirement of the THO complex for expression of intronless and intron-containing genes.
Figure 4.
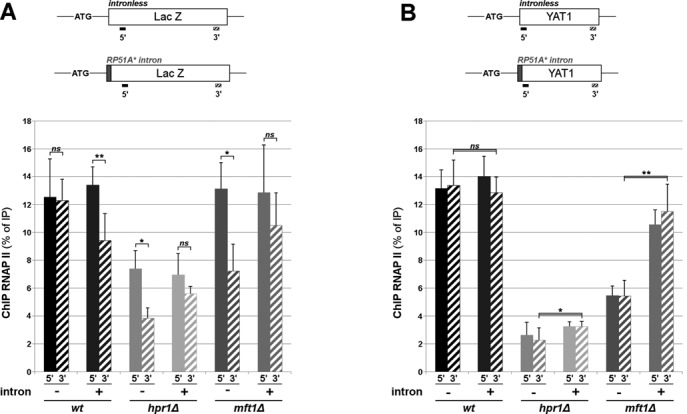
Intronic sequences can attenuate the transcriptional defects caused by THO complex inactivation. (A) RNAP II ChIP was performed in wt and mutant cells transformed with intronless or intron-containing centromeric LacZ reporters. Values (mean ± SD; n = 3) represent the ratios between immunoprecipitated and input DNA amounts. The positions of qPCR amplicons (5′, 299–385 bp and 3′, 2763–2911 bp) along the LacZ gene are indicated. (B) RNAP II ChIP was performed in wt and mutant cells transformed with intronless or intron-containing YAT1 reporters. Values (mean ± SD; n = 4) represent the ratios between immunoprecipitated and input DNA amounts. The positions of qPCR amplicons (5′, 33–132 bp and 3′, 1852–1951 bp) along the YAT1 gene are indicated. Specificity of the ChIP assay was confirmed by checking RNAP II recruitment onto an untranscribed, intergenic region (our unpublished results).
Nuclear pore mutants affecting Ulp1 localization exhibit gene expression defects
A phenotypic signature of THO mutants is therefore the differential reduction in intronless versus intron-containing LacZ reporter expression, observed both with ‘leakage’ and ‘splicing’ constructs (Supplementary Figure S1C). In agreement with the fact that Ulp1 targets the THO complex (Figure 1C), this signature was satisfyingly shared by the ulp1 mutant (Figure 5A,B and Supplementary Table S4). Of note, since Ulp1 was previously reported to regulate 2μ-plasmid levels (49), the 2μ-based intronless and intron-containing reporter constructs were checked to be similarly affected in ulp1 cells (Supplementary Figure S2C).
Figure 5.
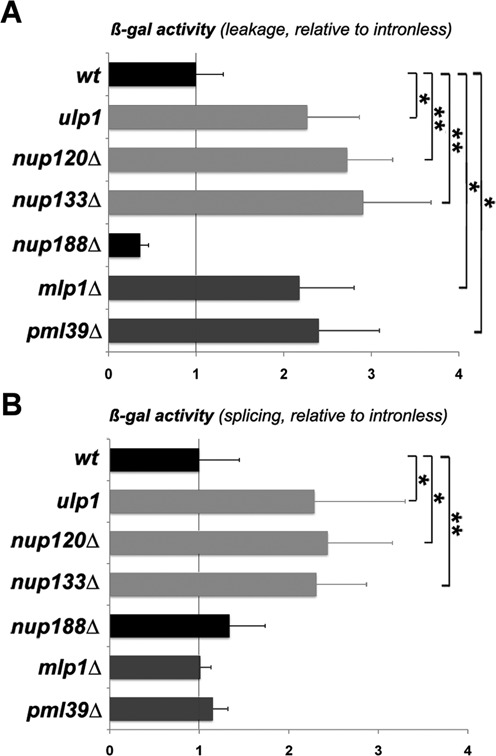
Nuclear pore mutants differentially affect intron-containing reporter expression. β-gal activities (mean ± SD; n = 4) were measured in the indicated strains transformed with intronless, ‘leakage’ (A), and ‘splicing’ (B) reporters. β-gal activities relative to the ones obtained with the intronless reporter are represented. Values were set to 1 for wt cells. Raw data are provided in Supplementary Table S4.
We decided to take advantage of these LacZ-based reporter assays to further discriminate between nuclear pore mutants affected in gene transcription versus bona fide pre-mRNA leakage mutants. Relative expressions of both ‘leakage’ and ‘splicing’ reporters were then analyzed in Nup84 complex mutants, which were previously shown to display decreased levels of Ulp1 at NPCs without detectable effect on nuclear basket assembly (21,50). This revealed that Nup84 complex mutants (e.g. nup120Δ and nup133Δ) were less affected for the expression of both intron-containing reporters as compared to the intronless one (Figure 5A,B and Supplementary Table S4), resembling tho mutants. In agreement with this observation, transcription elongation defects were previously scored on LacZ and YAT1 genes in Nup84 complex mutants (51). Collectively, these results show that loss of Ulp1 activity or localization is associated with LacZ expression defects that are rescued in the presence of an intron, rather than genuine pre-mRNA leakage (Supplementary Figure S1C).
Bona fide pre-mRNA leakage mutants do not affect Ulp1 activity toward mRNP assembly
These results prompted us to reevaluate whether other nuclear pore mutants previously reported to play a role in pre-mRNA retention, such as mlp1Δ and pml39Δ, also interfered with the Ulp1/THO pathway. We first checked whether mlp1/pml39 mutants would trigger pre-mRNA leakage as a possible consequence of Ulp1 mislocalization. Indeed, Mlp1, Pml39 and Ulp1 share a common localization at the nuclear basket of NPCs and a moderate decrease in Ulp1 association to NPCs was previously scored in mlp1Δ cells (49). Similarly, our microscopy analysis only revealed a mild decrease of the NPC-associated levels of a GFP-tagged version of Ulp1 in the absence of Mlp1, and no effect of the pml39Δ mutation (Figure 6A). In view of the reported interdependence between Ulp1 NPC localization and stability (49,50), we further examined the total amounts of Ulp1 in mlp1Δ and pml39Δ cells by western-blot analysis, which confirmed that unlike Nup84 complex mutants these mutations do not compromise Ulp1 levels (Figure 6Ba,b). Consistent with these observations, global analysis of SUMO conjugates did not reveal major changes in SUMO (de)conjugation or processing in mlp1Δ and pml39Δ cells, as opposed to mutants strongly impairing Ulp1 activity (e.g. ulp1)(Supplementary Figure S4).
Figure 6.

Bona fide pre-mRNA leakage mutants do not affect Ulp1 activity. (A) Fluorescence microscopy analysis of Ulp1-GFP in wt, mlp1Δ, or pml39Δ cells expressing Nup49-mCherryFP and grown at 30°C. Images of single-channel fluorescence for GFP and mCherry are shown (left) as well as the merge with differential interference contrast (DIC, right). Scale bar, 5 μm. (B) a: whole cell extracts of the indicated cell types grown at 30°C were analyzed by western blotting using anti-GFP antibodies (upper panel). Ponceau staining was used as loading control (lower panel). b: the relative amount of Ulp1-GFP was quantified from panel (a). Values (mean ± SD; n = 3) were normalized to the Ponceau signal and set to 1 for wt. (C) a: soluble extracts (left panel, ‘inputs’) and nuclear cap-binding complex (Cbc2-protA)-associated mRNPs (right panel, ‘IP’) isolated from the indicated strains were analyzed by immunoblotting using the indicated antibodies. Note that while Hpr1 association is solely affected in ulp1 cells, recruitment of the export receptor Mex67 is not altered in the analyzed mutants. b: the relative amounts of Hpr1 and Mex67 associated to Cbc2-bound mRNPs were quantified from panel (a). Values (mean ± SD; n = 3) were normalized to the amounts of immunoprecipitated Cbc2 and set to 1 for wt.
Since Ulp1 inactivation was previously reported to impair THO complex desumoylation, thereby lowering its association with nuclear mRNPs (19), we went on to analyze mRNP composition and THO complex recruitment in mlp1 and pml39 mutants. Unlike Ulp1 inactivation, loss of Mlp1 or Pml39 did not alter THO association with mRNPs pulled-down by the nuclear cap-binding complex (as probed by immunoblotting against the THO complex subunit Hpr1; Figure 6Ca,b). Taken together, these data demonstrate that nuclear pore mutants affecting pre-mRNA retention (e.g. mlp1Δ, pml39Δ) do not systematically affect Ulp1 localization and activity toward the THO complex. In agreement with these observations, both mlp1Δ and pml39Δ mutants exhibited bona fide pre-mRNA leakage with no effect on the expression of the ‘splicing’ reporter, as opposed to tho/ulp1/Nup84 complex mutants (Figure 5A,B; Supplementary Figure S1B).
Nuclear pore components influence distinct stages of intron-containing gene expression
This set of data suggests that Mlp1/Pml39 and Nup84 complex/Ulp1/THO impact on distinct stages of intron-containing mRNA biogenesis and export. To confirm this hypothesis, we performed epistasis analysis by combining both mlp1Δ and ulp1 mutations. Measurement of β-galactosidase activities from the ‘leakage’ reporter relative to the ones obtained from the intronless reporter revealed a synergic phenotype in the double mlp1Δ ulp1 mutant (Figure 7A and Supplementary Table S4), supporting the fact that both mutants interfere with the cytoplasmic expression of unspliced LacZ mRNAs through distinct mechanisms. To strengthen this finding, the same assay was performed in another ulp1 mutant strain carrying the ulp1-ΔN allele, which leads to Ulp1 mislocalization from the nuclear periphery (52). ulp1-ΔN cells exhibited increased relative β–gal activites from the ‘leakage’ reporter, confirming the results obtained with the thermosensitive ulp1 allele (Figure 7A). Noteworthy, mlp1Δ ulp1-ΔN cells also displayed a synergic effect on this phenotype, as observed for mlp1Δ ulp1 cells (Figure 7A). In addition, growth assay revealed a genetic interaction between mlp1Δ and either ulp1 or ulp1-ΔN mutations, as shown by the decreased viability of the double mlp1Δ ulp1 and mlp1Δ ulp1-ΔN mutants as compared to each of the mlp1Δ, ulp1 and ulp1-ΔN single mutants (Figure 7B). Finally, mild overexpression of ULP1 did not rescue pre-mRNA leakage in the mlp1Δ mutant (Figure 7C and Supplementary Table S4). Taken together, our data support a model in which these nuclear pore mutants affect intron-containing mRNA expression at two distinct stages (Figure 7D).
Figure 7.
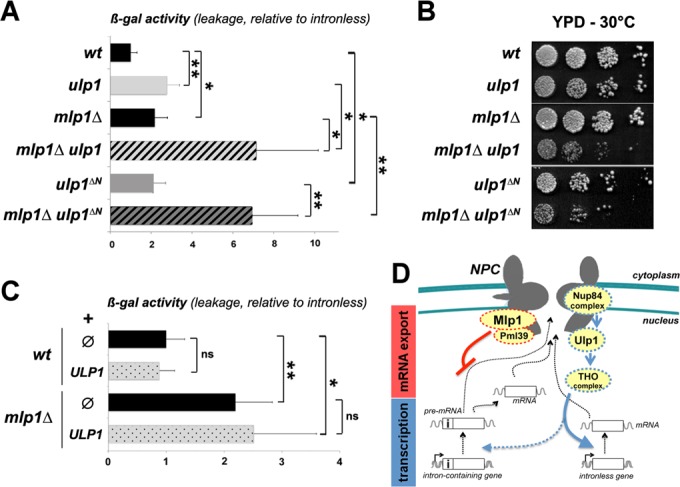
Distinct functions of nuclear pore-associated proteins in intron-containing gene expression. (A) β-gal activities (mean ± SD; n = 5) were measured in the indicated strains transformed with the pre-mRNA ‘leakage’ reporter. β-gal activities relative to the ones obtained with strains transformed with the intronless reporter are represented. Values were set to 1 for wt cells. (B) Serial dilutions of the indicated strains were grown at 30°C on YPD solid medium. (C) β-gal activities (mean ± SD; n = 5) were measured in the indicated strains transformed with the pre-mRNA ‘leakage’ reporter together with the ULP1-overexpressing construct (pRS315-NOP1-GFP-ULP1, ‘ULP1’) or an empty vector (pRS315, ‘ø’). β-gal activities relative to the ones obtained with strains transformed with the intronless reporter are represented. Values were set to 1 for wt cells without ULP1. Note that the ULP1 construct used here was previously reported to suppress the phenotypes associated with Ulp1 loss-of-function (49,50). Raw data are provided in Supplementary Table S4. (D) Mlp1/Pml39 and Nup84/Ulp1/THO impact on distinct stages of intron-containing mRNA biogenesis and export. On the one hand, the Nup84 complex contributes to the NPC localization of Ulp1, which targets the THO complex for desumoylation. Alteration in any of these components would impinge on THO complex activity, which is more dispensable for the transcription of intron-containing as compared to intronless genes. On the other hand, Mlp1/Pml39 have specific roles in nuclear retention of intron-containing pre-mRNAs (see Discussion). Note that nuclear pores also influence gene expression at the stage of transcription initiation, as shown for some inducible genes (40).
DISCUSSION
In this study, we have investigated the contribution of a number of mRNP and nuclear pore components to intron-containing gene expression in yeast (Figures 1B and 5A). The use of a battery of intron-containing reporter genes allowed us to discriminate between nuclear pore components such as the Nup84 complex and the SUMO protease Ulp1 on the one hand, that are differentially required for the transcription of intronless versus intron-containing genes, and nuclear basket-associated proteins Mlp1/Pml39 on the other hand, that prevent pre-mRNA export at a later stage (Supplementary Figure S1, Figure 7D).
It had been previously hypothesized that sumoylated target(s) of the SUMO protease Ulp1 could contribute to its reported function in pre-mRNA retention (5,25). Here, in agreement with our recent identification of the THO complex subunit Hpr1 as a sumoylated target of Ulp1 (19), our epistasis analysis of ulp1 and tho mutants reveals that they trigger the same apparent ‘pre-mRNA leakage’ phenotype through a common mechanism (Figure 1B,C). However, we show that mutants affecting the THO complex (hpr1Δ, mft1Δ, hpr1-K60–65R) and Ulp1 activity (ulp1) or localization (ulp1-ΔN, nup133Δ, nup120Δ) differentially impair the expression of intronless and intron-containing reporters, rather than triggering bona fide pre-mRNA leakage : indeed, (i) the same differential reduction of intron-containing and intronless reporter expression is observed regardless of the frame of the intron with the LacZ coding sequence, including when the pre-mRNA does not encode β-gal (Figure 2B); (ii) this differential inhibition is also detectable at the level of total mRNAs, regardless of their nuclear or cytoplasmic localization (Figure 2D). The fact that this phenotype had not been detected in a previous study using the same reporters in a tho mutant (28) is likely due to the low sensitivity of the colorimetric assay (30) as compared to our chemiluminescent assay. Notably, our observations are in line with the well-established functions of the THO complex as an early mRNP assembly factor whose proper recruitment, by preventing DNA-mRNA hybrid formation, precludes transcriptional defects and genetic instability (4).
Unexpectedly, our data also reveal that the presence of an intron can partially prevent this deleterious impact of THO inactivation on mRNA expression. This effect was observed for distinct natural introns (Figure 3A–C) and was not restricted to the LacZ heterologous gene: indeed, insertion of an intron in the natural THO target gene YAT1 partially rescues the mRNA expression defects arising in tho mutants (Figure 3D–F). Consistent with the reported effects of THO inactivation on RNAP II transcription, we further found that the intron exerts its alleviating effect by partially restoring proper RNAP II occupancy on the LacZ and YAT1 genes (Figure 4A–B). Of note, the presence of the intron only partially rescues the strong drop in RNAP II recruitment and mRNA expression in the hpr1Δ mutant (Figures 2D, 3E, 4A,B). However, the intron almost fully suppresses the milder YAT1 mRNA expression and RNAP II occupancy defects in mft1Δ cells (Figures 3E and 4B). These last results support the fact that transcription is most likely the main step in the gene expression process that is impaired by THO inactivation and impacted by the presence of the intron, although we cannot rule out that other post-transcriptional stages may be similarly affected. Different molecular mechanisms could account for the protective effect of the intron against THO complex inactivation. One possibility is that early spliceosome recruitment and/or splicing of such 5′-located introns could trigger alternative mRNP assembly pathways in tho mutants, thereby rescuing mRNA biogenesis defects. Alternatively, the effect of the intron could be related to its positive impact on gene looping, and therefore on mRNA synthesis (53). Finally, since decreasing the rate of transcription was previously reported to rescue THO deficiencies (54–57), the presence of the intron could slow down transcription, rendering mRNP biogenesis less sensitive to THO inactivation. While such explanation could account for the effect of the intron in the LacZ gene, in which intron insertion was detected to affect RNAP II occupancy in wt cells (Figure 4A), it is unlikely to provide a general rationale for our observations: indeed, in wt cells, the presence of the intron in the YAT1 gene does not appear to dampen RNAP II recruitment. Additional studies will now be required to further discriminate between these non-exclusive hypotheses.
Among other nuclear pore components, Ulp1 does not appear to play a bona fide role in mRNA quality control since the apparent ‘pre-mRNA leakage’ observed in ulp1/tho mutants is attributable to defective expression of the reporters. In agreement with our conclusions, ulp1 mutants do not exhibit genetic interactions with mutants of the QC-related endonuclease Swt1, as opposed to MLP1 inactivation (27). In addition, our previous proteomic analysis of Mlp2-associated mRNPs demonstrated that mRNP recruitment at NPCs does not require Ulp1 function (19). This docking at NPCs likely represents a true quality control step as notably revealed by the mRNA export defects caused by disruption of the Nab2-Mlp1 interaction (35), or associated with the formation of intranuclear Nab2-Mlp1 foci formed upon heat shock or Pml39 overexpression (58,21). Mlp1–2 proteins would not directly influence mRNP composition, consistent with our findings (Figure 6C), but would either selectively commit spliced mRNAs for nuclear export or retain unspliced transcripts, as previously hypothesized (6). How this selection is operated remains however to be understood.
The two pathways highlighted by our epistasis analysis (Figure 7A,B) illustrate the multiple contributions of nuclear pore proteins to mRNA expression: the Mlp1/Pml39 pathway essentially acts on mRNA quality control, whereas Ulp1 contributes to optimal mRNA synthesis through THO complex sumoylation (Figure 7D). Of note, Ulp1 activity is expected to modulate gene expression through additional sumoylated targets. So far, the only other identified mRNA biogenesis factor targeted by Ulp1 is the general corepressor Ssn6, whose desumoylation was shown to contribute to the derepression of some inducible loci (52). In the future, extensive identification of Ulp1 and SUMO targets in the mRNA biogenesis machinery will certainly provide further clues on the intricate relationships between nuclear pores and gene expression.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
Acknowledgments
We are very grateful to C. Petibon for technical help; to A. Corbett, C. Dargemont, M. Fromont-Racine, M. Hochstrasser, E. Hurt, A. Jacquier, S. Chavez, F. Stutz and X. Zhao for sharing reagents; to V. Doye and F. Stutz for critical reading of the manuscript; and to V. Doye for discussions during the course of this work.
FUNDING
CNRS, Fondation ARC pour la Recherche sur le Cancer [Projet ARC to B.P.]; Ligue Nationale contre le Cancer [Comité d'Ile de France to B.P.]. H.B was the recipient of PhD fellowships from Ministère de l'Enseignement Supérieur et de la Recherche and Fondation ARC pour la Recherche sur le Cancer. A.B. was the recipient of a post-doctoral fellowship from Ligue Nationale contre le Cancer. Funding for open access charge: CNRS/Fondation ARC pour la Recherche sur le Cancer.
Conflict of interest statement. None declared.
REFERENCES
- 1.Oeffinger M., Zenklusen D. To the pore and through the pore: A story of mRNA export kinetics. Biochim. Biophys. Acta. 2012;1819:494–506. doi: 10.1016/j.bbagrm.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller-McNicoll M., Neugebauer K.M. How cells get the message: dynamic assembly and function of mRNA-protein complexes. Nat. Rev. Genet. 2013;14:275–287. doi: 10.1038/nrg3434. [DOI] [PubMed] [Google Scholar]
- 3.Nino C.A., Herissant L., Babour A., Dargemont C. mRNA nuclear export in yeast. Chem. Rev. 2013;113:8523–8545. doi: 10.1021/cr400002g. [DOI] [PubMed] [Google Scholar]
- 4.Luna R., Rondon A.G., Aguilera A. New clues to understand the role of THO and other functionally related factors in mRNP biogenesis. Biochim. Biophys. Acta. 2012;1819:514–520. doi: 10.1016/j.bbagrm.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Tutucci E., Stutz F. Keeping mRNPs in check during assembly and nuclear export. Nat. Rev. Mol. Cell Biol. 2011;12:377–384. doi: 10.1038/nrm3119. [DOI] [PubMed] [Google Scholar]
- 6.Fasken M.B., Corbett A.H. Mechanisms of nuclear mRNA quality control. RNA Biol. 2009;6:237–241. doi: 10.4161/rna.6.3.8330. [DOI] [PubMed] [Google Scholar]
- 7.Schmid M., Jensen T.H. Transcription-associated quality control of mRNP. Biochim. Biophys. Acta. 2013;1829:158–168. doi: 10.1016/j.bbagrm.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Schmid M., Jensen T.H. The exosome: a multipurpose RNA-decay machine. Trends Biochem. Sci. 2008;33:501–510. doi: 10.1016/j.tibs.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Libri D., Dower K., Boulay J., Thomsen R., Rosbash M., Jensen T.H. Interactions between mRNA export commitment, 3′-end quality control, and nuclear degradation. Mol. Cell. Biol. 2002;22:8254–8266. doi: 10.1128/MCB.22.23.8254-8266.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rougemaille M., Gudipati R.K., Olesen J.R., Thomsen R., Seraphin B., Libri D., Jensen T.H. Dissecting mechanisms of nuclear mRNA surveillance in THO/sub2 complex mutants. EMBO J. 2007;26:2317–2326. doi: 10.1038/sj.emboj.7601669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assenholt J., Mouaikel J., Andersen K.R., Brodersen D.E., Libri D., Jensen T.H. Exonucleolysis is required for nuclear mRNA quality control in yeast THO mutants. RNA. 2008;14:2305–2313. doi: 10.1261/rna.1108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnet A., Palancade B. Regulation of mRNA trafficking by nuclear pore complexes. Genes (Basel) 2014;5:767–791. doi: 10.3390/genes5030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyle J.H., Bor Y.C., Rekosh D., Hammarskjold M.L. The Tpr protein regulates export of mRNAs with retained introns that traffic through the Nxf1 pathway. RNA. 2011;17:1344–1356. doi: 10.1261/rna.2616111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajanala K., Nandicoori V.K. Localization of nucleoporin Tpr to the nuclear pore complex is essential for Tpr mediated regulation of the export of unspliced RNA. PLoS One. 2012;7:e29921. doi: 10.1371/journal.pone.0029921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yap K., Lim Z.Q., Khandelia P., Friedman B., Makeyev E.V. Coordinated regulation of neuronal mRNA steady-state levels through developmentally controlled intron retention. Genes Dev. 2012;26:1209–1223. doi: 10.1101/gad.188037.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green D.M., Johnson C.P., Hagan H., Corbett A.H. The C-terminal domain of myosin-like protein 1 (Mlp1p) is a docking site for heterogeneous nuclear ribonucleoproteins that are required for mRNA export. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1010–1015. doi: 10.1073/pnas.0336594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinciguerra P., Iglesias N., Camblong J., Zenklusen D., Stutz F. Perinuclear Mlp proteins downregulate gene expression in response to a defect in mRNA export. EMBO J. 2005;24:813–823. doi: 10.1038/sj.emboj.7600527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niepel M., Molloy K.R., Williams R., Farr J.C., Meinema A.C., Vecchietti N., Cristea I.M., Chait B.T., Rout M.P., Strambio-De-Castillia C. The nuclear basket proteins Mlp1p and Mlp2p are part of a dynamic interactome including Esc1p and the proteasome. Mol. Biol. Cell. 2013;24:3920–3938. doi: 10.1091/mbc.E13-07-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bretes H., Rouviere J.O., Leger T., Oeffinger M., Devaux F., Doye V., Palancade B. Sumoylation of the THO complex regulates the biogenesis of a subset of mRNPs. Nucleic Acids Res. 2014;42:5043–5058. doi: 10.1093/nar/gku124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galy V., Gadal O., Fromont-Racine M., Romano A., Jacquier A., Nehrbass U. Nuclear retention of unspliced mRNAs in yeast is mediated by perinuclear Mlp1. Cell. 2004;116:63–73. doi: 10.1016/s0092-8674(03)01026-2. [DOI] [PubMed] [Google Scholar]
- 21.Palancade B., Zuccolo M., Loeillet S., Nicolas A., Doye V. Pml39, a novel protein of the nuclear periphery required for nuclear retention of improper messenger ribonucleoparticles. Mol. Biol. Cell. 2005;16:5258–5268. doi: 10.1091/mbc.E05-06-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iglesias N., Tutucci E., Gwizdek C., Vinciguerra P., Von Dach E., Corbett A.H., Dargemont C., Stutz F. Ubiquitin-mediated mRNP dynamics and surveillance prior to budding yeast mRNA export. Genes Dev. 2010;24:1927–1938. doi: 10.1101/gad.583310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braunschweig U., Barbosa-Morais N.L., Pan Q., Nachman E.N., Alipanahi B., Gonatopoulos-Pournatzis T., Frey B., Irimia M., Blencowe B.J. Widespread intron retention in mammals functionally tunes transcriptomes. Genome Res. 2014;24:1774–1786. doi: 10.1101/gr.177790.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ares M. Jr, Grate L., Pauling M.H. A handful of intron-containing genes produces the lion's share of yeast mRNA. RNA. 1999;5:1138–1139. doi: 10.1017/s1355838299991379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis A., Felberbaum R., Hochstrasser M. A nuclear envelope protein linking nuclear pore basket assembly, SUMO protease regulation, and mRNA surveillance. J. Cell Biol. 2007;178:813–827. doi: 10.1083/jcb.200702154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dziembowski A., Ventura A.P., Rutz B., Caspary F., Faux C., Halgand F., Laprevote O., Seraphin B. Proteomic analysis identifies a new complex required for nuclear pre-mRNA retention and splicing. EMBO J. 2004;23:4847–4856. doi: 10.1038/sj.emboj.7600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skruzny M., Schneider C., Racz A., Weng J., Tollervey D., Hurt E. An endoribonuclease functionally linked to perinuclear mRNP quality control associates with the nuclear pore complexes. PLoS Biol. 2009;7:e8. doi: 10.1371/journal.pbio.1000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hackmann A., Wu H., Schneider U.M., Meyer K., Jung K., Krebber H. Quality control of spliced mRNAs requires the shuttling SR proteins Gbp2 and Hrb1. Nat. Commun. 2014;5:3123. doi: 10.1038/ncomms4123. [DOI] [PubMed] [Google Scholar]
- 29.Palancade B., Doye V. Sumoylating and desumoylating enzymes at nuclear pores: underpinning their unexpected duties. Trends. Cell. Biol. 2008;18:174–183. doi: 10.1016/j.tcb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Bronstein I., Martin C.S., Fortin J.J., Olesen C.E., Voyta J.C. Chemiluminescence: sensitive detection technology for reporter gene assays. Clin. Chem. 1996;42:1542–1546. [PubMed] [Google Scholar]
- 31.Ulrich H.D., Davies A.A. In vivo detection and characterization of sumoylation targets in Saccharomyces cerevisiae. Methods Mol. Biol. 2009;497:81–103. doi: 10.1007/978-1-59745-566-4_6. [DOI] [PubMed] [Google Scholar]
- 32.Gwizdek C., Hobeika M., Kus B., Ossareh-Nazari B., Dargemont C., Rodriguez M.S. The mRNA nuclear export factor Hpr1 is regulated by Rsp5-mediated ubiquitylation. J. Biol. Chem. 2005;280:13401–13405. doi: 10.1074/jbc.C500040200. [DOI] [PubMed] [Google Scholar]
- 33.Luna R., Jimeno S., Marin M., Huertas P., Garcia-Rubio M., Aguilera A. Interdependence between transcription and mRNP processing and export, and its impact on genetic stability. Mol. Cell. 2005;18:711–722. doi: 10.1016/j.molcel.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Segref A., Sharma K., Doye V., Hellwig A., Huber J., Luhrmann R., Hurt E. Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. EMBO J. 1997;16:3256–3271. doi: 10.1093/emboj/16.11.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fasken M.B., Stewart M., Corbett A.H. Functional significance of the interaction between the mRNA-binding protein, Nab2, and the nuclear pore-associated protein, Mlp1, in mRNA export. J. Biol. Chem. 2008;283:27130–27143. doi: 10.1074/jbc.M803649200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Navarro S., Fischer T., Luo M.J., Antunez O., Brettschneider S., Lechner J., Perez-Ortin J.E., Reed R., Hurt E. Sus1, a functional component of the SAGA histone acetylase complex and the nuclear pore-associated mRNA export machinery. Cell. 2004;116:75–86. doi: 10.1016/s0092-8674(03)01025-0. [DOI] [PubMed] [Google Scholar]
- 37.Faza M.B., Kemmler S., Jimeno S., Gonzalez-Aguilera C., Aguilera A., Hurt E., Panse V.G. Sem1 is a functional component of the nuclear pore complex-associated messenger RNA export machinery. J. Cell Biol. 2009;184:833–846. doi: 10.1083/jcb.200810059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chavez S., Beilharz T., Rondon A.G., Erdjument-Bromage H., Tempst P., Svejstrup J.Q., Lithgow T., Aguilera A. A protein complex containing Tho2, Hpr1, Mft1 and a novel protein, Thp2, connects transcription elongation with mitotic recombination in Saccharomyces cerevisiae. EMBO J. 2000;19:5824–5834. doi: 10.1093/emboj/19.21.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Rubio M., Chavez S., Huertas P., Tous C., Jimeno S., Luna R., Aguilera A. Different physiological relevance of yeast THO/TREX subunits in gene expression and genome integrity. Mol. Genet. Genomics. 2008;279:123–132. doi: 10.1007/s00438-007-0301-6. [DOI] [PubMed] [Google Scholar]
- 40.Chavez S., Garcia-Rubio M., Prado F., Aguilera A. Hpr1 is preferentially required for transcription of either long or G+C-rich DNA sequences in Saccharomyces cerevisiae. Mol. Cell. Biol. 2001;21:7054–7064. doi: 10.1128/MCB.21.20.7054-7064.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chavez S., Aguilera A. The yeast HPR1 gene has a functional role in transcriptional elongation that uncovers a novel source of genome instability. Genes Dev. 1997;11:3459–3470. doi: 10.1101/gad.11.24.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Legrain P., Rosbash M. Some cis- and trans-acting mutants for splicing target pre-mRNA to the cytoplasm. Cell. 1989;57:573–583. doi: 10.1016/0092-8674(89)90127-x. [DOI] [PubMed] [Google Scholar]
- 43.Cheng T.H., Chang C.R., Joy P., Yablok S., Gartenberg M.R. Controlling gene expression in yeast by inducible site-specific recombination. Nucleic Acids Res. 2000;28:E108. doi: 10.1093/nar/28.24.e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yofe I., Zafrir Z., Blau R., Schuldiner M., Tuller T., Shapiro E., Ben-Yehezkel T. Accurate, model-based tuning of synthetic gene expression using introns in S. cerevisiae. PLoS Genet. 2014;10:e1004407. doi: 10.1371/journal.pgen.1004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mason P.B., Struhl K. Distinction and relationship between elongation rate and processivity of RNA polymerase II in vivo. Mol. Cell. 2005;17:831–840. doi: 10.1016/j.molcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 46.Voynov V., Verstrepen K.J., Jansen A., Runner V.M., Buratowski S., Fink G.R. Genes with internal repeats require the THO complex for transcription. Proc. Natl. Acad. Sci. U.S.A. 2006;103:14423–14428. doi: 10.1073/pnas.0606546103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huertas P., Garcia-Rubio M.L., Wellinger R.E., Luna R., Aguilera A. An hpr1 point mutation that impairs transcription and mRNP biogenesis without increasing recombination. Mol. Cell. Biol. 2006;26:7451–7465. doi: 10.1128/MCB.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rondon A.G., Jimeno S., Garcia-Rubio M., Aguilera A. Molecular evidence that the eukaryotic THO/TREX complex is required for efficient transcription elongation. J. Biol. Chem. 2003;278:39037–39043. doi: 10.1074/jbc.M305718200. [DOI] [PubMed] [Google Scholar]
- 49.Zhao X., Wu C.Y., Blobel G. Mlp-dependent anchorage and stabilization of a desumoylating enzyme is required to prevent clonal lethality. J. Cell Biol. 2004;167:605–611. doi: 10.1083/jcb.200405168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palancade B., Liu X., Garcia-Rubio M., Aguilera A., Zhao X., Doye V. Nucleoporins prevent DNA damage accumulation by modulating Ulp1-dependent sumoylation processes. Mol. Biol. Cell. 2007;18:2912–2923. doi: 10.1091/mbc.E07-02-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tous C., Rondon A.G., Garcia-Rubio M., Gonzalez-Aguilera C., Luna R., Aguilera A. A novel assay identifies transcript elongation roles for the Nup84 complex and RNA processing factors. EMBO J. 2011;30:1953–1964. doi: 10.1038/emboj.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Texari L., Dieppois G., Vinciguerra P., Contreras M.P., Groner A., Letourneau A., Stutz F. The nuclear pore regulates GAL1 gene transcription by controlling the localization of the SUMO protease Ulp1. Mol. Cell. 2013;51:807–818. doi: 10.1016/j.molcel.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 53.Moabbi A.M., Agarwal N., El Kaderi B., Ansari A. Role for gene looping in intron-mediated enhancement of transcription. Proc. Natl. Acad. Sci. U.S.A. 2012;109:8505–8510. doi: 10.1073/pnas.1112400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jensen T.H., Boulay J., Olesen J.R., Colin J., Weyler M., Libri D. Modulation of transcription affects mRNP quality. Mol. Cell. 2004;16:235–244. doi: 10.1016/j.molcel.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 55.Jimeno S., Garcia-Rubio M., Luna R., Aguilera A. A reduction in RNA polymerase II initiation rate suppresses hyper-recombination and transcription-elongation impairment of THO mutants. Mol. Genet. Genomics. 2008;280:327–336. doi: 10.1007/s00438-008-0368-8. [DOI] [PubMed] [Google Scholar]
- 56.Jimeno S., Tous C., Garcia-Rubio M.L., Ranes M., Gonzalez-Aguilera C., Marin A., Aguilera A. New suppressors of THO mutations identify Thp3 (Ypr045c)-Csn12 as a protein complex involved in transcription elongation. Mol. Cell. Biol. 2011;31:674–685. doi: 10.1128/MCB.01188-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mouaikel J., Causse S.Z., Rougemaille M., Daubenton-Carafa Y., Blugeon C., Lemoine S., Devaux F., Darzacq X., Libri D. High-frequency promoter firing links THO complex function to heavy chromatin formation. Cell Rep. 2013;5:1082–1094. doi: 10.1016/j.celrep.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Carmody S.R., Tran E.J., Apponi L.H., Corbett A.H., Wente S.R. The mitogen-activated protein kinase Slt2 regulates nuclear retention of non-heat shock mRNAs during heat shock-induced stress. Mol. Cell. Biol. 2010;30:5168–5179. doi: 10.1128/MCB.00735-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


