Figure 4.
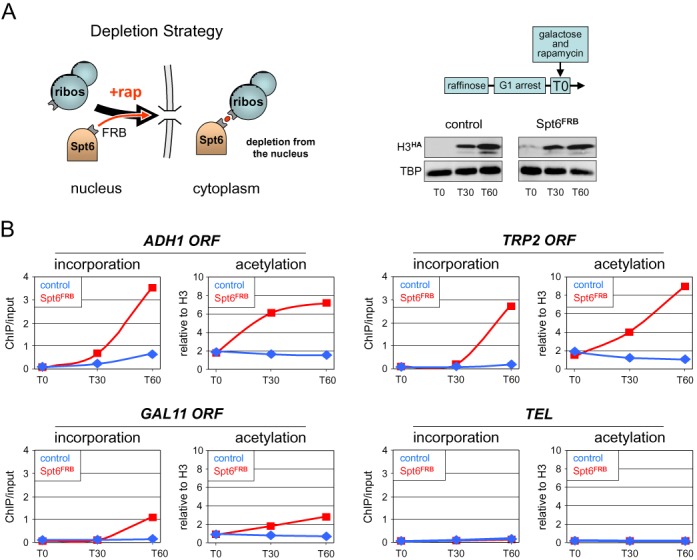
Spt6 inactivation leads to increased histone incorporation and H3K9 acetylation within transcribed regions. (A) Schematic illustration of the anchor-away strategy. Upon addition of rapamycin (+ rap, red dot), Spt6 fused to the rapamycin-binding domain FRB is rapidly exported out of the nucleus through its interaction with a ribosomal protein carrying the complementary FKBP rapamycin-binding domain (for details, see (25)). Right panel: experimental diagram. Cells from a Spt6-anchor-away strain (Spt6FRB) containing the galactose-inducible H3HA construct and from an isogenic control strain (control) lacking the Spt6FRB fusion were arrested in G1 by alpha factor. At T0, galactose and rapamycin were added to both cultures to, respectively, induce H3HA expression and deplete Spt6FRB from the nucleus. Shown on the lower right is a western blot analysis of H3HA expression and TBP as a control in the two strains. (B) The amounts of newly incorporated H3HA (left panels) and the levels of H3K9ac (right panels) at the indicated ORFs were monitored in the Spt6-anchor-away (Spt6FRB, red lines) and control (control, blue lines) strains by quantitative ChIP using antibodies against the HA tag or against H3K9ac. Data for H3HA incorporation are expressed as the percentage of input DNA recovered. The ChIP signals for H3K9ac were normalized to those obtained using a core histone H3 antibody for each genomic region to account for variations in nucleosome occupancy during the experiment and between different loci. TEL is a subtelomeric heterochromatin region on chromosome VI where Spt6 depletion is without consequence. An independent biological experiment is shown in Supplementary Figure S7.
