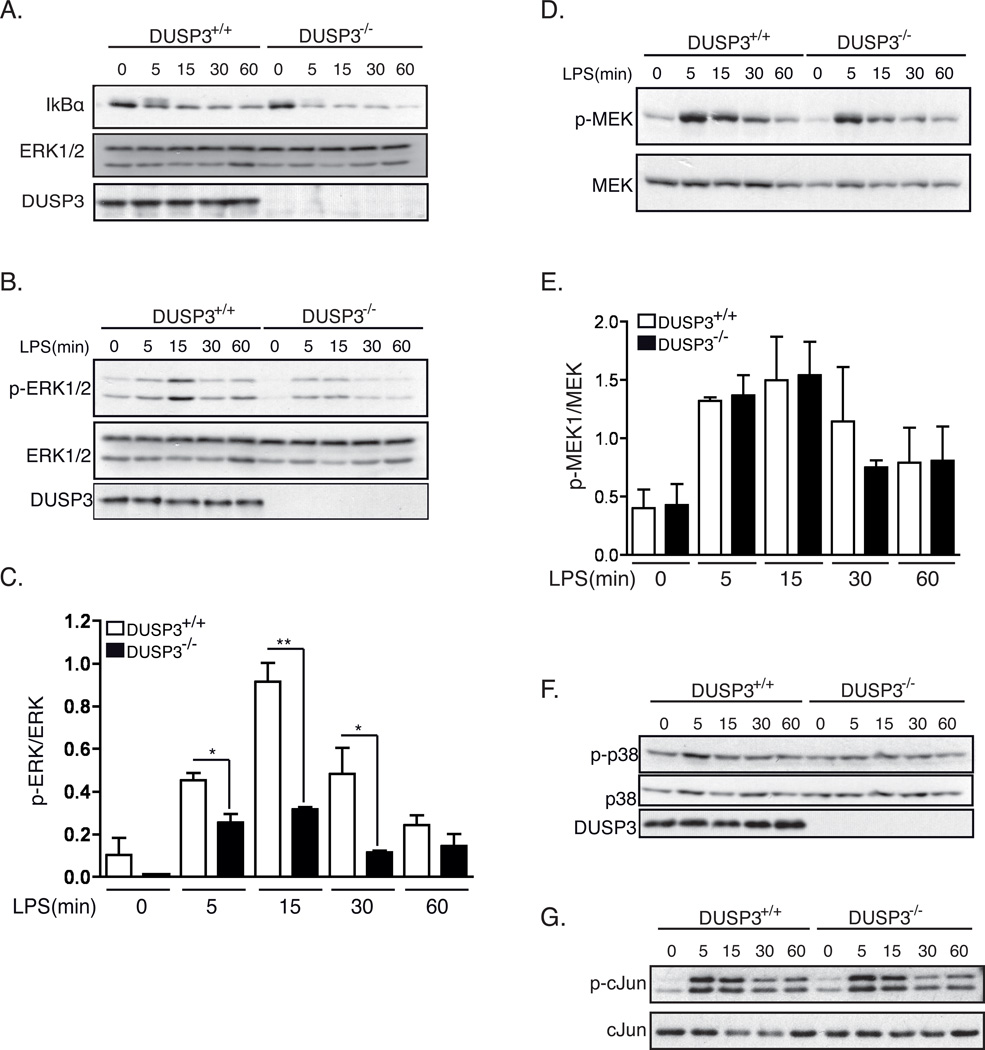
Figure 9. DUSP3 deficiency affects ERK1/2 phosphorylation but not JNK, p38 nor NF-κB pathways after TLR4 triggering.
PMs isolated from 10–12 weeks old DUSP3+/+ and DUSP3−/− mice were stimulated ex vivo with 1µg/mL of LPS for the indicated time points. Equal amount of proteins were loaded on SDS-PAGE and western blots were performed using (A) anti-IκB antibody and anti-ERK1/2 as a loading control; (B) anti-phospho-ERK1/2 (Thr202/Tyr204) and anti-ERK1/2 as a loading control; (D) anti-phospho-MEK1/2 (Ser217/221) and anti-MEK1/2; anti-phospho-p38 (Thr180/Tyr182) and (F) anti-p38. (C and E) Densitometry quantification of p-ERK1/2/ERK1/2 (B) and p-MEK/MEK (D) were performed using Imaje J software. Results are presented as a ratio of p-ERK/ERK and p-MEK/MEK from 3 independent experiments. Data are shown as mean ± SEM. *, p<0,05, **, p<0,01 determined using unpaired t-test. (G) JNK activity assay. Phospho-SAPK/JNK (Thr183/Tyr185) was immunoprecipitated from non-activated and LPS activated PMs lysates. Recombinant c-Jun was used as a substrate for the kinase assay. JNK activity was revealed using anti-phospho-c-Jun (Ser63). Representative JNK assay is shown.