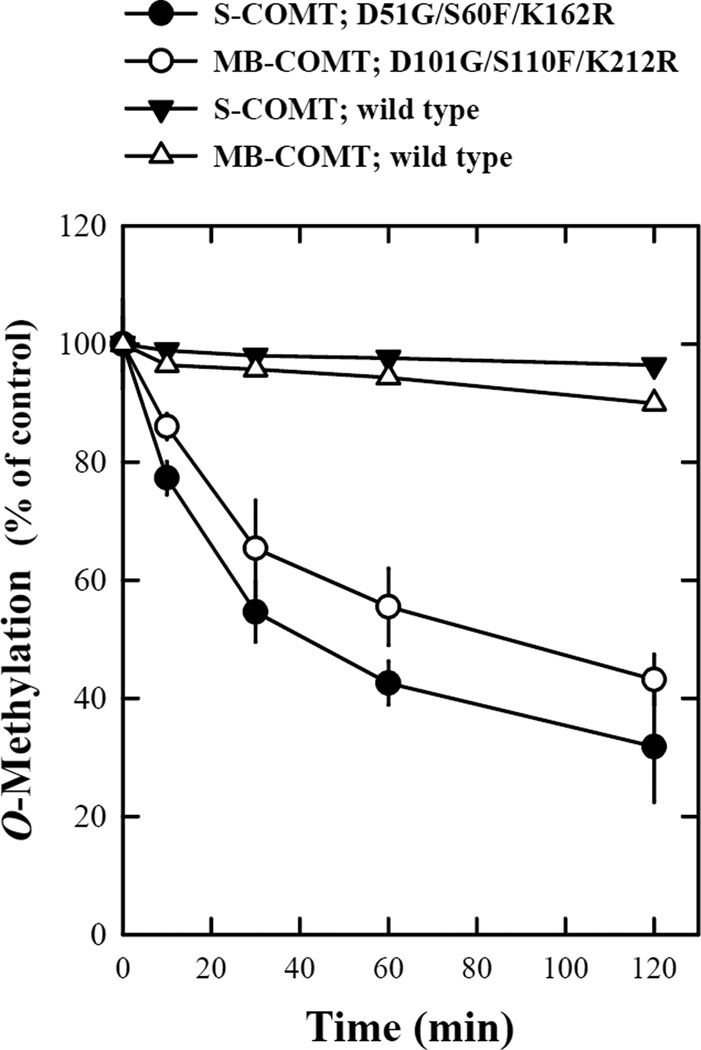
Figure 5.
Stabilities of the wild-type and mutant human recombinant S- and MB-COMTs. The reaction mixtures consisted of the recombinant COMT protein (at 16.2 µg/mL for S-COMT or 17.1 µg/mL for MB-COMT), 1.2 mM MgCl2, 100 µM AdoMet (containing 0.5–1 mCi [methyl-3H]AdoMet), 2-OH-E2 as a substrate and 1 mM 1,4-dithiothreitol in Tris-HCl buffer (50 mM, pH 7.4). The final volume of the reaction mixture was usually 300 µL. The reaction was initiated by addition of the recombinant human COMT protein and carried out at 37°C for 15 minutes. To test the thermostability of the mutant and wild-type COMTs, the enzymes were first preincubated at 37°C for the indicated length of time immediately before testing their catalytic activity for the O-methylation of 2-OH-E2 (at 10 µM). Each value is the mean ± S.D. of triplicate measurements.