Figure 4.
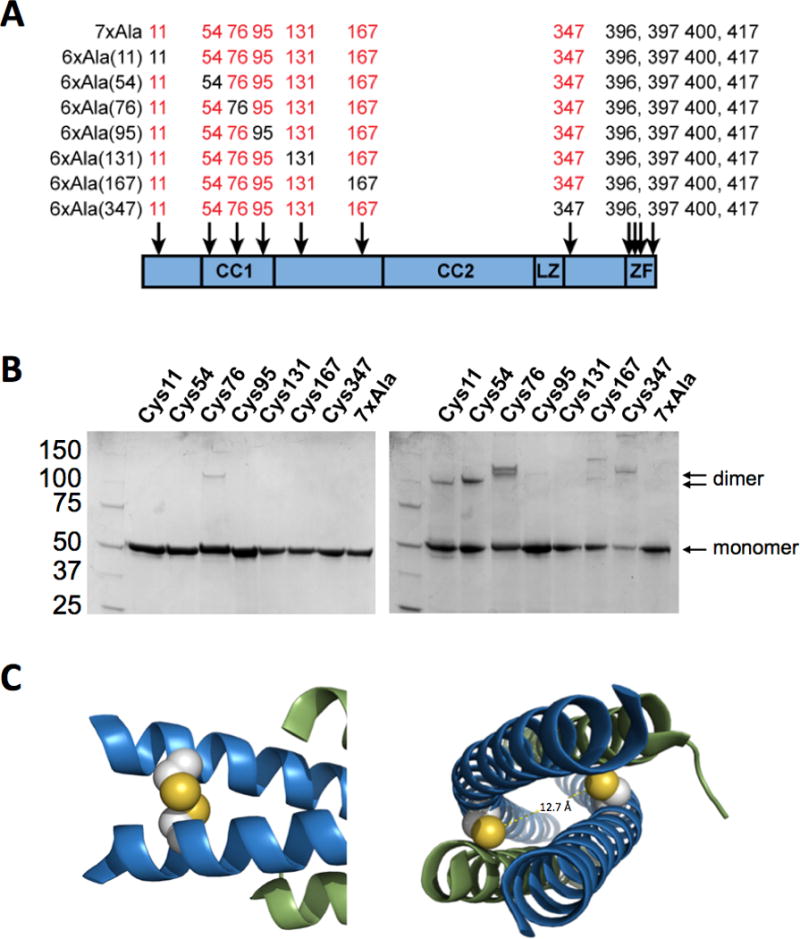
Role of individual cysteine residues in NEMO dimerization and aggregation. (A) Panel of 6xAla NEMO mutants in which each of the cysteines in the region of NEMO sequence between residue 1 and 395 has been individually added back into the 7xAla construct. (B) SDS-PAGE analysis of the 6xAla mutant panel under reducing (left) and non-reducing (right) conditions, showing that the mutants containing cysteines at positions 11, 54, 76 and 347 show a propensity to form disulfide-linked covalent dimers. (C) Images of the published co-crystal structure of NEMO(44-111) with IKKβ(701-745) (30), showing that the cysteines at position 54 (left) are well-positioned to form an inter-chain disulfide bond in the active conformation of the protein, while the cysteines at position 76 are not as their sulfur atoms are separated by 12.7 Å.
