Fig 2.
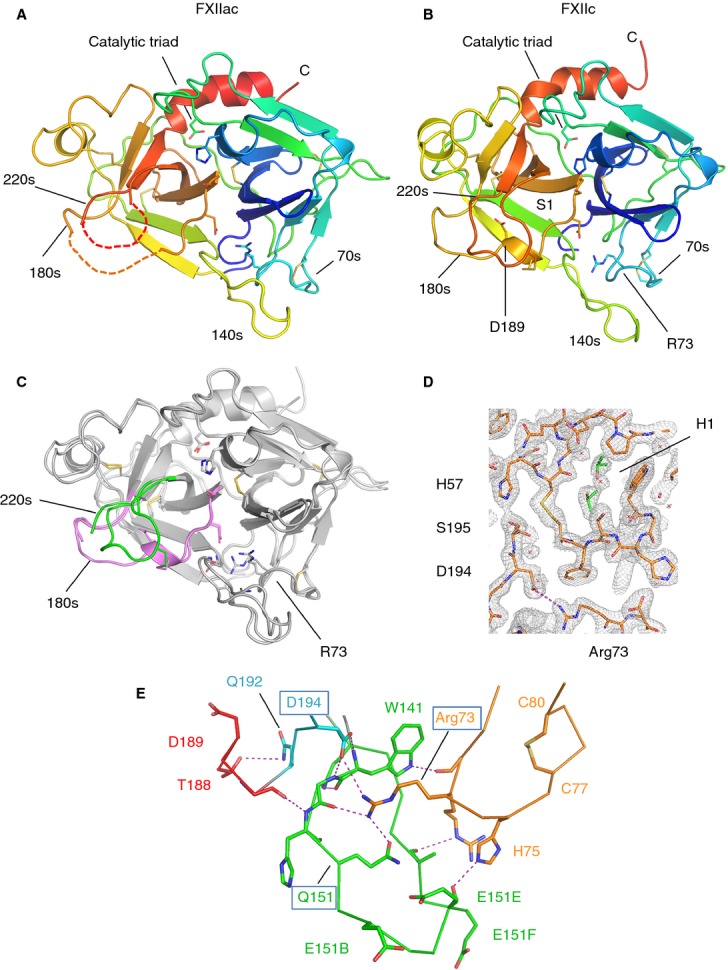
FXII protease structures. (A) Cartoon diagram of the FXIIac topology colored from the N-terminus to the C-terminus, with the N-terminal β-barrel in blue/green and the C-terminal β-barrel in yellow/orange. Loops containing residue ranges derived from the chymotrypsin sequence numbering are labeled 70s, 140s, 180s, and 220s. Disulfide bonds are shown in yellow. Regions not visible in the electron density are shown as dotted lines. (B) Cartoon diagram of the FXIIc structure. (C) FXIIac and FXIIc crystal structures superposed. The two structures are represented as cartoon traces with the 180-loop colored pink and the 220-loop colored green. (D) Electron density map (2Fo – Fc coefficients) for the final refined coordinates of FXIIc at 2.1-Å resolution shown in the area of the active site residue Ser195 contoured at 1.5 root mean square deviation. Isopropanol molecules bound in the H1 pocket are shown in green as sticks, and water molecules as red crosses. (E) Interactions between loops from the two FXIIc β-barrels are shown, illustrating how Arg73 connects to residues from both the 180-loop and 140-loop. The 80-loop, 140-loop and 220-loop are colored orange, green, and cyan, respectively, with S1 helix residues in red. Electrostatic and hydrogen-bonding interactions are shown as purple dotted lines. The positively charged residues Arg72, Arg73 and His75 from the 70-loop are shown as sticks in orange.
