Figure 3. Redox cycles of hemoglobin and its transition into higher oxidation state (pseudoperoxidase) and subsequent oxidative changes.
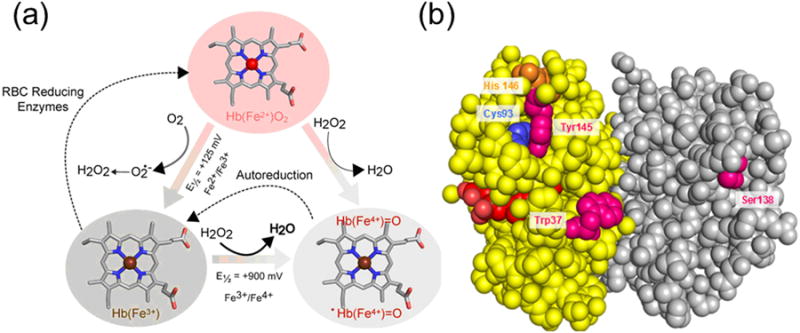
A) Pseudoperoxidase cycle Heme iron, a transition metal within the heme prosthetic group of Hb undergoes spontaneous oxidation from ferrous to ferric oxidation states. This process indirectly produces H2O2, which can further react with ferric and ferrous Hb to produce ferryl species and its protein cationic radical. Thermodynamically, these reactions are driven by the redox potentials of each pair. B) Protein-heme oxidative modifications and oxidation of amino acids. Hb Beta subunits (yellow) bear the burden of the radical chemistry as a handful of amino acids in the “hotspots” are targeted and are irreversibly oxidized. In addition, heme-to-protein linkages are formed which will ultimately result in heme loss.
