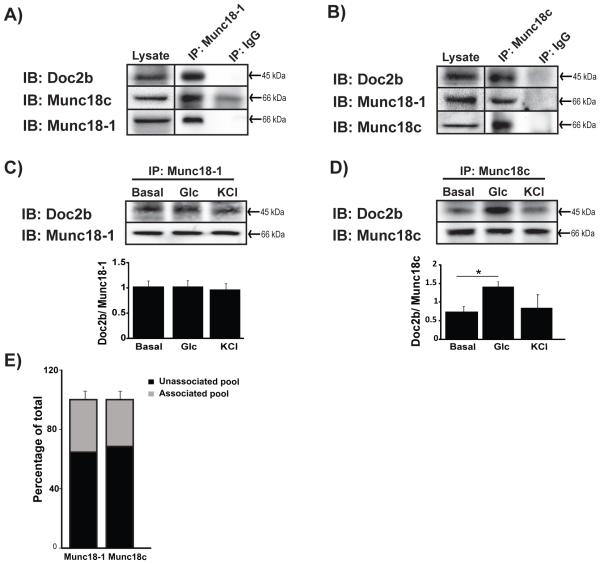
Figure 1. Interaction between Munc18-1, Doc2b and Munc18c in MIN6 beta cells.
Cleared detergent lysates prepared from MIN6 cells that were stimulated with glucose for 5 min were immunoprecipitated (IP) with mouse anti-Munc18-1 (A) or rabbit anti-Munc18c (B) antibodies. Coprecipitated proteins were resolved by 10% SDS-PAGE for immunoblot detection (IB). IgG was used to control for antibody specificity. Input proteins were taken from each reaction to confirm the presence of each protein in the lysates. Data are representative of at least 3 independent sets of beta cell lysates. Vertical lines indicate splicing of lanes from within the same gel exposure. MIN6 cells left unstimulated (basal) or stimulated for 5 min with glucose or KCl were used for immunoprecipation with anti-Munc18-1 (C) or anti-Munc18c (D) and Doc2b co-precipitated was quantified from three independent experiments. Bar graphs show the average ± SE; *p<0.05 versus basal. (E) Optical density quantitation of three independent GST-Doc2b interaction assays using MIN6 cell lysates. Pre, prior to GST-Doc2b addition; Post, flow-through following 2 h incubation and pelleting of GST-Doc2b beads. The unassociated pool was determined by comparing the quantity of Munc18 proteins in the flow-through to that in the input (prior to GST-Doc2b addition).