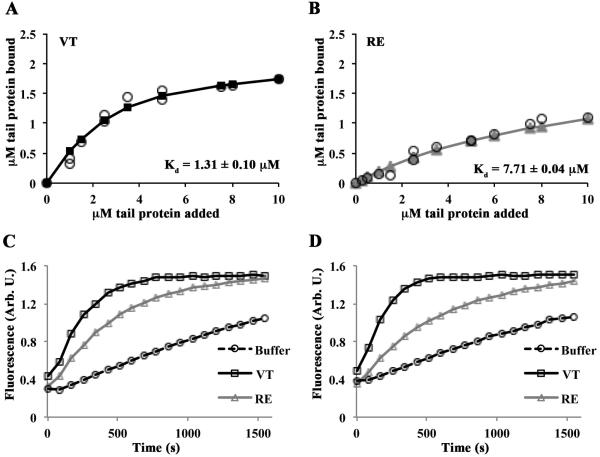
Figure 2. RE is an actin binding and polymerization mutant in physiological salt conditions.
(A, B) VT and RE bind actin with different affinities. Reactions containing VT or RE and G-actin were polymerized by the addition of F-salts, then centrifuged at 80,000 rpm to pellet actin filaments. Twenty-five percent of the supernatant and the entire pellets were run on separate gels. The amount of tail protein in the pellet of cosedimentation reactions was plotted as a function of the tail concentration added to the sample. Estimates of Kd were generated by fitting the data to the quadratic binding equation and are noted in each panel. The error on the Kd is the error of the fit. Open circles represent individual observations; solid lines with squares (VT; A) or triangles (RE; B) represent the fitted data. (C,D) Varying concentrations of VT, RE, or an equivalent volume of buffer was added to 1µM pyrene labeled G-actin in G-buffer, then polymerized with F-salts, and the change in fluorescence was monitored over time. Representative graphs are shown. RE fails to stimulate actin polymerization to the same extent as VT at 0.25µM (C) or 0.5µM (D).