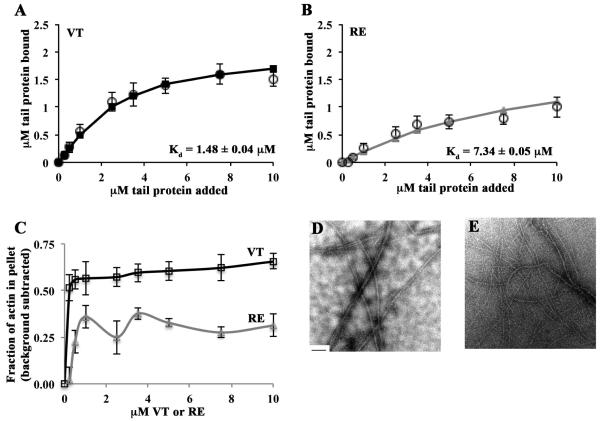
Figure 3. RE is an actin bundling mutant in physiological salt conditions.
(A, B) RE does not bind or bundle actin as well as VT. Reactions containing VT or RE and G-actin were polymerized by the addition of F-salts, then centrifuged at 20,000 rpm to pellet actin bundles. Twenty-five percent of the supernatant and the entire pellets were run on separate gels. The amount of tail protein recovered in the pellet was plotted as a function of the tail concentration added to the sample. Estimates of Kd were generated by fitting the data to the quadratic binding equation and are noted in each panel. Open circles represent data from at least 3 independent experiments. Error bars are SEM. Solid lines with squares (VT; A) or triangles (RE; B) represent the fitted data. The error on the Kd is the error of the fit. (C) RE does not bundle actin as well as VT. The amount of actin recovered in the pellet was plotted as a function of the total tail concentration added to the sample. The percent of actin that was pelleted in the control experiment containing only actin was subtracted from each data point. Shown is the mean of at least 3 independent experiments ± SEM. (D) VT bundles actin in physiological salt conditions. VT (2.5µM) and actin (1µM) were combined then polymerized by the addition of F-salts. A fraction of the reactions was spotted on Formvar-coated TEM grids and imaged at 12,000x magnification. Bundles several filaments thick are visible. (E) RE does not bundle actin as well as VT. When RE (2.5µM) and actin (1µM) were combined, thinner and more loosely packed bundles than those in (D) result. The scale bar in (D) is 200nm.