Abstract
Purpose
To establish optimal intensity-modulated radiation therapy (IMRT) techniques for treating the left breast and regional nodes, using moderate deep-inspiration breath hold.
Methods and Materials
We developed four IMRT plans of differing complexity for each of 10 patients following lumpectomy for left breast cancer. A dose of 60 Gy was prescribed to the boost planning target volume (PTV) and 52.2 Gy to the breast and supraclavicular, infraclavicular, and internal mammary nodes. Two plans used inverse-planned beamlet techniques: a 9-field technique, with nine equispaced axial beams, and a tangential beamlet technique, with three to five ipsilateral beams. The third plan (a segmental technique) used a forward-planned multi-segment technique, and the fourth plan (a segmental blocked technique) was identical but included a block to limit heart dose. Dose–volume histograms were generated, and metrics chosen for comparison were analyzed using the paired t test.
Results
Mean heart and left anterior descending coronary artery doses were similar with the tangential beamlet and segmental blocked techniques but higher with the segmental and 9-field techniques (mean paired difference of 15.1 Gy between segmental and tangential beamlet techniques, p < 0.001). Substantial volumes of contralateral tissue received dose with the 9-field technique (mean right breast V2, 58.9%; mean right lung V2, 75.3%). Minimum dose to ≥95% of breast PTV was, on average, 45.9 Gy with tangential beamlet, 45.0 Gy with segmental blocked, 51.4 Gy with segmental, and 50.2 Gy with 9-field techniques. Coverage of the internal mammary region was substantially better with the two beamlet techniques than with the segmental blocked technique.
Conclusions
Compared to the 9-field beamlet and segmental techniques, a tangential beamlet IMRT technique reduced exposure to normal tissues and maintained reasonable target coverage.
Keywords: Breast cancer, IMRT, Radiotherapy, Treatment planning, Dosimetry
INTRODUCTION
Intensity-modulated radiation therapy (IMRT) is a technique in which radiation is administered via multiple individual segments of varying intensities, rather than through traditional radiation fields of uniform intensities or modulated through wedges alone. IMRT techniques vary in sophistication, ranging from simple, manual division of clinically chosen beams into a few large segments, with the aim of improving dose homogeneity, to extremely complex techniques using computer-assisted inverse planning to develop conformal plans from many small individual beamlets.
In breast cancer treatment, standard doses have yielded high rates of tumor control in the adjuvant setting (1), and IMRT has been directed largely at minimizing toxicity (2–4). Indeed, while radiotherapy is now established as an integral part of the management of early-stage breast cancer, both for patients undergoing breast-conserving therapy and those with sufficient risk of residual locoregional disease after mastectomy (1), concerns remain regarding its long-term toxicities, including potential effects upon cosmetic outcomes due to inhomogeneity of dose within breast tissue, as well as potential toxicities related to incidental exposure of the underlying lung and heart.
Thus far, in breast cancer treatment, intensity modulation has primarily been studied in the clinical setting as a means by which to improve dose homogeneity within the breast (5, 6), rather than to spare underlying structures. The division of tangential beams into a small number of relatively large segments results in improvements in dose homogeneity within the breast and has been proposed as a feasible and efficient method of treatment (7, 8). Recent trials have demonstrated that the use of this simple form of intensity modulation to improve dose homogeneity in the breast results in clinically appreciable benefits in terms of reduced skin and soft tissue toxicity compared with two-dimensional treatment planning (2–4).
Although simple, segmental techniques have been demonstrated to provide benefits in reducing soft tissue toxicity, there may also be a role for more sophisticated beamlet IMRT techniques in the treatment of breast cancer. As demonstrated in other cancer disease sites, sophisticated optimization techniques can be a powerful tool for improving dose conformality to spare critical adjacent normal tissues. Given the concerning association between RT and ischemic heart disease (9–11), further investigation to identify optimal IMRT techniques for reducing cardiac exposure in the context of breast cancer treatment is warranted. After all, although contemporary RT exposes substantially less heart volume to radiation than earlier techniques, computed tomography (CT)-based analyses still show delivery of high doses to small regions of the left anterior heart, including the left coronary vasculature. Cardiac perfusion defects have been documented even in patients treated in recent years with advanced three-dimensional planning techniques, although the clinical consequences of these defects are not yet clear (12). Moreover, potential interactions between cardiotoxic systemic agents such as doxorubicin and trastuzumab and RT must be considered (13, 14). Since there are no known “safe” levels of radiation to the heart, especially in the presence of systemic therapy, techniques that further minimize cardiac exposure to RT are important subjects for further exploration.
It is especially important to consider IMRT techniques when the target includes the regional lymph node basins to which breast cancer may spread. Treatment of the regional nodes and especially the internal mammary nodes (IMNs), increases the complexity of RT delivery and, with certain techniques, may increase the risks of cardiac and pulmonary toxicity. Many radiation oncologists have incorporated nodal therapy into their treatment of node-positive patients both after mastectomy and after breast conservation because the landmark Danish (15) and Canadian (16) postmastectomy trials targeted the regional lymph nodes in addition to the chest wall, despite the fact that trials specifically assessing the benefit of nodal RT (17, 18) have yet to mature.
Investigators at several institutions have reported promising initial experiences with IMRT planning for coverage of the breast and regional nodes in patients with breast cancer. Our group at the University of Michigan reported a treatment planning study assessing an inverse-planned beamlet technique of IMRT treatment to the postmastectomy chest wall and regional nodes. In that planning study of 10 left-sided breast cancer patients after mastectomy, Krueger and colleagues found that a nine-field technique, using high-powered cost functions and normal tissue complication probability-based costlets, achieved full target coverage while delivering doses to the heart and ipsilateral lung that were similar to those delivered via conventional techniques (19). However, given increased low-dose irradiation of the contralateral breast and contralateral lung observed with this IMRT technique and concerns about second malignancies, that study concluded that while this represented an important first step toward developing an IMRT plan for breast/chest wall and nodal tissue, doses to contralateral nontarget structures would need to be reduced prior to consideration of clinical application. Nevertheless, other investigators have continued to pursue nine-field approaches and have even suggested that such techniques are appropriate for use in the clinical setting (20).
In this study, we sought to establish optimal IMRT techniques for treating the left breast and regional nodes by using moderately deep inspiration breath hold by comparing four possible techniques. In this way, we evaluated more fully the trade-offs between IMRT techniques of varying complexities.
METHODS AND MATERIALS
Ten patients with left-sided stage II or III breast cancer, who underwent breast-conserving surgery and who were enrolled in an institutional review board-approved prospective study, were selected for this treatment planning study. Patients were of various body habitus. During preparation for treatment, all patients underwent contrast-enhanced CT scanning on a SinMed model breast board, with arms raised, at deep inspiration (75%), using an active breathing control device (21). Three-millimeter slices were obtained from the neck through to the upper abdomen and transferred to the in-house treatment planning system.
Targets included the breast, supraclavicular nodes, infraclavicular nodes, and superior internal mammary nodes (interspaces 1–3), as defined by the treating attending radiation oncologist using an atlas for guidance (22). The breast borders were defined clinically by placing radiopaque catheters on the patients’ skin as described previously (19). Clinical target volumes and planning target volumes (PTVs) were defined using expansions based on previous measurements assessing the reproducibility of target position, using active breathing control combined with in-house measurements assessing the accuracy of daily positioning for the breast (21). For consistency, all structures were confined to 5 mm from the external surface of the patient. Contours of all relevant normal tissues, including contralateral breast, whole heart, left anterior descending (LAD) coronary artery, left lung, right lung, left brachial plexus, and spinal cord were approved by the treating physician and specified as structures to avoid. An additional structure specified as “external” encompassed all normal tissues not otherwise specified (such as the arm, posterior thorax, and soft tissues) by subtracting the volumes of targets and specified normal tissues from the external surface.
Four plans to deliver 60 Gy to the boost PTV and 52.2 Gy in 30 fractions to the whole breast and regional nodes were developed for each patient. Two plans used inverse-planned beamlet IMRT techniques and delivered a simultaneous boost, and the other two plans used forward-planned multisegment techniques. One technique (the “9-field technique”) used nine equally spaced fields around each patient in the axial plane, as described previously (19). Cost functions placed high priority upon avoidance of contralateral breast and lung to reduce beamlet intensities through the contralateral breast, lung, and other normal tissues. All beams used 6 MV photons divided into 1- × 1-cm segments or “beamlets.” An in-house in verse planning system determined the intensity of each beamlet, as described in our previous work (23).
The second beamlet IMRT technique (the “tangential beamlet technique”) used beam angles selected by the physician and dosimetrist. This technique used a smaller number of intensity-modulated photon beams from angles similar to those commonly used in three-dimensional conformal radiotherapy, along with supplementary dose from a small number of electron fields where necessary. More specifically, the primary photon beam angles used to treat the breast or chest wall target volume were tangentially oriented. An additional photon beam was added over the supraclavicular region, using a single isocentric technique. Table 1 presents detailed information regarding the number and types of beams used in each patient with this technique. Three to five photon beam angles were used for the supraclavicular, medial, and lateral fields. Three of 10 patients had an additional photon field specifically for the tumor bed. Five of the 10 patients also had electron beams included in their plans with this technique. Electrons were used to spare the heart, treat the internal mammary nodes, or treat the boost region. To achieve an integrated treatment plan for all targets and to minimize plan sensitivity to motion, beamlets were deliberately overlapped (for 1-3 cm) in the inferior portion of the supraclavicular field with the superior region of the medial field. The optimization was used to derive the appropriate intensity for all photon fields rather than using a field-matching approach that could result in hot or cold spots. In some plans, additional beams were added if necessary to provide target coverage while improving sparing of normal tissues. These additional beams included an additional photon or electron beam that was located more anteriorly over the boost volume and/or an additional electron beam over the internal mammary region and part of the medial portion of the breast to deliver dose while sparing dose to the heart. When electron beams were used, the optimization was done with the electron plan delivering a background dose. This further supported an integrated treatment plan for delivering dose to all targets.
Table 1.
Plans developed with the tangential beamlet technique
| No. of photon beams and use |
|||
|---|---|---|---|
| Patient | Supraclavicular/medial/lateral | Boost | Electron beams and justification |
| 1 | 3 | ||
| 2 | 3 | 9 and 12 MeV for IMN; 6 MeV heart; 6 MeV boost | |
| 3 | 5 | ||
| 4 | 3 | 1 | 9 MeV for IMN and heart |
| 5 | 3 | 1 | |
| 6 | 3 | 1 | 9 MeV for IMN |
| 7 | 5 | 9 MeV for heart sparing | |
| 8 | 3 | ||
| 9 | 3 | ||
| 10 | 3 | 9 MeV for IMN and heart sparing | |
Abbreviation: IMN = internal mammary nodal region.
The third and fourth plans were created using a forward-planned multisegmental technique, similar to that described by investigators at William Beaumont Hospital (24). First, open-field tangent beams were weighted to deliver approximately 80% of the tangential dose. Then, isodose surfaces were defined to aid in the selection of segments. Two to four segments were added to increase the homogeneity across the targets. These techniques used a single isocenter with a matched area at the infraclavicular region. For all patients, the boost region was planned with a conventional en face electron or a planned photon boost. Table 2 provides further details of the beam energy and number of segments used for each patient for the segmental techniques. The third plan (segmental) was created with an emphasis on obtaining the best target coverage. The fourth plan (segmental blocked) was created with the same beam angles but with the heart blocked from the tangential fields. For this plan, an electron beam was added when necessary to supplement the target doses.
Table 2.
Plans developed with the segmental techniques
| Patient | Energy of supraclavicular photon fields | Segmentation and energy of medial tangents | Segmentation and energy of lateral tangents | Mechanisms of tumor bed coverage |
|---|---|---|---|---|
| 1 | 16 MV | 5 6MV segments | 3 6MV segments | 9 MeV |
| 2 | 6 MV | 3 6MV segments | 3 6MV segments | 9 MeV |
| 3 | 6 MV | 5 6MV segments | 4 6MV segments | 9 MeV |
| 4 | 16 MV | 6 6MV segments | 4 6MV segments | 12 MeV |
| 5 | 16 MV | 4 6MV segments | 6 6MV segments | 9 MeV |
| 6 | 6 MV | 4 6MV segments | 4 6MV segments | 12 MeV |
| 7 | 16 MV | 2 gantry angles with 7 6MV segments | 2 gantry angles: 3 6MV segments plus 1 16 MV | 6 MV/16 MV photons |
| 8 | 16 MV | 4 6MV segments | 4 16 MV segments | 16 MV photons |
| 9 | 6 MV | 3 6MV segments plus 1 16MV | 3 6MV segments plus 1 16MV | 16 MV photons |
| 10 | 16 MV | 4 16MV segments | 4 16MV segments | 6 MV/16 MV photons |
For all plans, treatment planning goals were articulated through a treatment planning directive. This directive specified objectives of treating 95% of the breast and nodal target volumes to 52.2 Gy. Other objectives were dose homogeneity for the boost and whole breast; heart and LAD maximum dose <15 Gy and mean dose <5 Gy; and left lunch V20<33%. The spinal cord maximum dose was not to exceed 10 Gy, nor were the ipsilateral carotid or brachial plexus maximum doses to exceed 55 Gy. Additional constraints for the 9-field technique were to minimize dose to the contralateral breast (maximum dose of <3.9 Gy and mean dose of ≥0.3 Gy) and contralateral lung (maximum dose of <19.77 Gy and mean dose of ≥0.3 Gy). These additional criteria were determined after calculating contralateral doses typically received with traditional tangential techniques.
For analysis, dose–volume histograms were generated for all relevant structures for all techniques. A number of metrics were chosen to compare the techniques. Specifically, the mean dose, minimum dose, and maximum dose to each target structure were analyzed. The minimum dose was evaluated by considering the lowest dose received by at least 95% of the target volume (D95) and the maximum dose by considering the highest dose received by at least 1% of the target volume (Dmax). Clinically relevant doses to normal tissues were also assessed, including low doses that might potentially be relevant in terms of radiation-induced carcinogenesis.
Treatment plans were compared using the paired t test, with the tangential beamlet plan as the reference. Mean differences were calculated after subtracting the tangential beamlet plan values for each metric in each patient from the values for the same metric in the same patient for the plan being compared. p values of ≥ 0.05 were considered significant.
RESULTS
Median patient age was 51 years old, ranging from 38 to 62 years. Median weight was 168 pounds and ranged from a minimum of 126 to a maximum of 239 pounds. Eight patients were white, and 2 were African American.
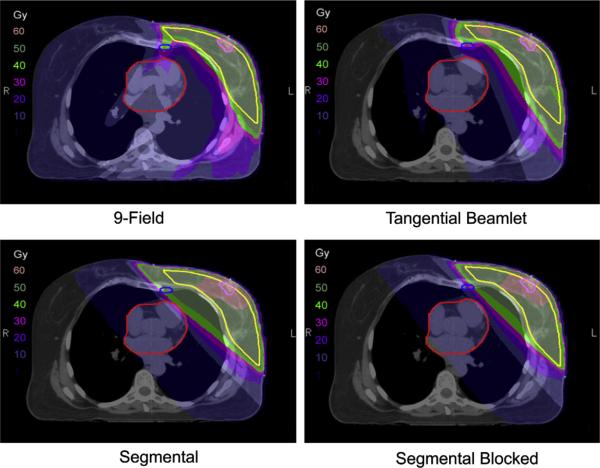
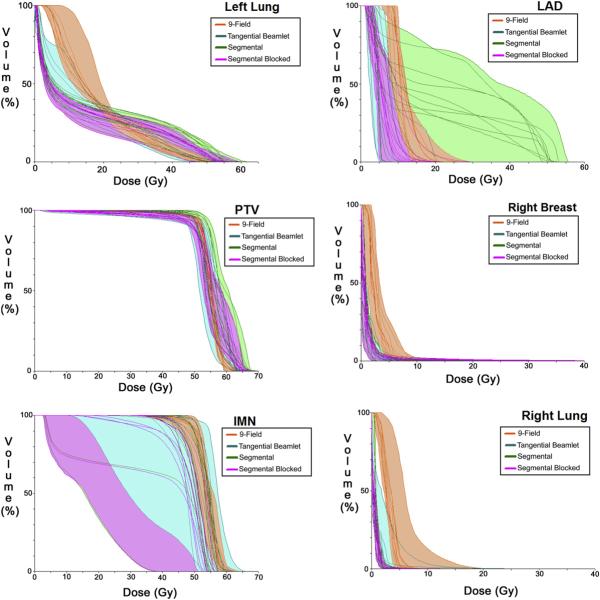
Figure 1 shows the dose distributions obtained with each technique in a representative patient. The example shows how the tangential beamlet and segmental heart-blocked techniques result in substantial decreases in the low-dose exposure of contralateral tissues compared to the 9-field technique and substantial decreases in high-dose exposure of ipsilateral tissues compared to the segmental technique. Figure 2 shows dose–volume histograms for all 10 patients for each technique for a number of key targets and critical normal structures.
Fig. 1.
Dose distributions on axial images from one representative patient.
Fig. 2.
Dose–volume histograms in all 10 patients with each technique for key target and normal structures. LAD = left anterior descending coronary artery; PTV = whole-breast planning target volume; IMN = internal mammary nodal planning target volume; orange = 9-field; aqua = tangential beamlet; green = segmental; pink = segmental blocked.
Table 3 summarizes the mean doses received by target and normal tissues in all 10 patients with each technique. Table 4 shows comparisons between the 9-field and tangential beam-let techniques, between the segmental and tangential beamlet techniques, and between the segmental blocked and tangential beamlet techniques. As shown in these tables, the primary differences between the techniques were in normal tissue doses.
Table 3.
Dosimetric parameters by technique
| Structure | Mean estimate for 9-field technique (SD) | Mean estimate for segmental technique (SD) | Mean estimate for heart blocked segmental technique (SD) | Mean estimate for tangential beamlet technique (SD) |
|---|---|---|---|---|
| Normal tissues | ||||
| Right lung | ||||
| Mean dose (Gy) | 3.18 (1.27) | 0.54 (0.23) | 0.44 (0.15) | 0.72 (0.67) |
| V2 (%) | 75.32 (13.36) | 3.30 (2.58) | 2.40 (1.95) | 10.34 (13.58) |
| V5 (%) | 11.09 (21.30) | 0.28 (0.26) | 0.15 (0.20) | 1.90 (5.19) |
| V10 (%) | 1.32 (4.09) | 0.03 (0.05) | 0.03 (0.05) | 0.38 (0.98) |
| Right breast/chest wall | ||||
| Mean dose (Gy) | 2.68 (0.74) | 1.01 (0.45) | 0.84 (0.38) | 0.65 (0.33) |
| V2 (%) | 58.93 (22.01) | 11.77 (6.22) | 9.11 (5.02) | 10.55 (8.08) |
| V5 (%) | 10.56 (8.27) | 2.28 (1.40) | 1.85 (1.27) | 0.77 (0.73) |
| V10 (%) | 0.46 (0.63) | 1.09 (0.75) | 0.94 (0.70) | 0.38 (0.41) |
| Left lung | ||||
| Mean dose (Gy) | 17.95 (1.44) | 15.68 (2.30) | 13.77 (1.91) | 13.99 (2.06) |
| V2 (%) | 99.94 (0.11) | 68.23 (4.34) | 65.79 (3.98) | 75.13 (9.57) |
| V5 (%) | 93.45 (5.14) | 49.17 (4.10) | 46.17 (3.93) | 54.00 (10.28) |
| V10 (%) | 67.80 (12.07) | 38.79 (4.60) | 35.76 (4.24) | 42.48 (10.23) |
| V20 (%) | 34.46 (4.20) | 30.82 (5.17) | 27.61 (4.50) | 29.49 (4.70) |
| V30 (%) | 17.76 (3.17) | 25.76 (5.21) | 22.24 (4.43) | 20.01 (4.50) |
| V40 (%) | 7.31 (2.31) | 19.44 (4.99) | 15.10 (4.35) | 9.80 (4.99) |
| V50 (%) | 0.89 (0.79) | 8.05 (3.47) | 3.82 (1.96) | 2.06 (1.93) |
| Heart | ||||
| Mean dose (Gy) | 7.18 (1.21) | 4.10 (1.46) | 1.91 (0.33) | 2.60 (1.33) |
| V2 (%) | 99.94 (0.20) | 47.76 (14.70) | 37.63 (12.71) | 51.47 (31.87) |
| V5 (%) | 80.18 (16.63) | 12.47 (6.18) | 3.40 (1.33) | 10.15 (13.27) |
| V10 (%) | 11.49 (7.32) | 7.11 (4.10) | 0.55 (0.39) | 2.46 (3.32) |
| V20 (%) | 0.31 (0.36) | 4.84 (3.11) | 0.03 (0.06) | 0.40 (0.79) |
| V30 (%) | 0.01 (0.02) | 3.34 (2.38) | 0.005 (0.01) | 0.05 (0.17) |
| LAD artery | ||||
| Mean dose (Gy) | 11.23 (1.53) | 20.59 (8.46) | 6.00 (1.63) | 5.44 (2.38) |
| Max dose to 1% volume (Gy) | 19.32 (4.29) | 46.60 (14.50) | 14.22 (5.34) | 9.81 (5.17) |
| Targets | ||||
| Whole breast PTV | ||||
| Mean dose (Gy) | 54.65 (0.41) | 57.22 (1.94) | 54.82 (1.74) | 54.52 (1.71) |
| D95 (Gy) | 50.24 (0.88) | 51.41 (1.88) | 44.97 (3.76) | 45.86 (4.77) |
| Max dose to 1% volume (Gy) | 61.39 (1.24) | 66.00 (1.43) | 65.14 (1.14) | 63.20 (0.96) |
| V47.5 (%) | 96.77 (1.06) | 98.57 (1.19) | 93.92 (1.82) | 94.50 (2.69) |
| Internal mammary region | ||||
| Mean dose (Gy) | 53.57 (1.22) | 45.95 (11.85) | 43.88 (11.15) | 49.40 (8.46) |
| D95 (Gy) | 47.98 (2.49) | 36.03 (17.28) | 30.61 (15.61) | 43.76 (10.93) |
| Max dose to 1% volume (Gy) | 59.60 (1.46) | 53.69 (6.48) | 52.46 (6.12) | 57.47 (5.47) |
| V47.5 (%) | 95.03 (4.86) | 72.71 (29.07) | 61.55 (25.96) | 79.75 (28.33) |
| Supraclavicular region | ||||
| Mean dose (Gy) | 53.78 (0.55) | 53.88 (2.19) | 52.56 (1.38) | 53.20 (1.75) |
| D95 (Gy) | 49.37 (2.57) | 49.88 (3.73) | 48.75 (3.31) | 49.43 (2.92) |
| Max dose to 1% volume (Gy) | 58.13 (0.91) | 57.43 (2.83) | 55.85 (1.88) | 58.15 (2.59) |
| Infraclavicular region | ||||
| Mean dose (Gy) | 53.45 (0.75) | 52.46 (2.33) | 50.72 (1.86) | 52.87 (1.95) |
| D95 (Gy) | 49.51 (1.66) | 46.38 (4.50) | 44.11 (3.18) | 49.41 (2.54) |
| Max dose to 1% volume (Gy) | 57.46 (0.47) | 57.46 (2.22) | 55.44 (1.76) | 57.27 (2.01) |
| Boost volume | ||||
| Mean dose (Gy) | 60.39 (1.20) | 63.84 (1.40) | 62.90 (1.32) | 61.26 (0.85) |
| D95 (Gy) | 58.79 (0.96) | 59.34 (6.14) | 60.58 (1.39) | 59.65 (1.41) |
| Max dose to 1% volume (Gy) | 62.41 (1.61) | 66.59 (1.65) | 65.62 (1.47) | 63.72 (0.69) |
| V47.5 (%) | 99.44 (0.79) | 99.88 (0.38) | 99.95 (0.13) | 99.58 (0.85) |
Abbreviations: V2 = percentage of the volume receiving 2 Gy or more, e.g., V5 is the percentage of the volume receiving 5 Gy or more, etc.; D95 = lowest dose received by at least 95% of the volume; SD = standard deviation.
Table 4.
Comparison of dosimetric parameters between techniques
| 9-field estimate minus tangential beamlet estimate |
Segmental estimate minus tangential beamlet estimate) |
Segmental blocked estimate minus tangential beamlet estimate |
||||
|---|---|---|---|---|---|---|
| Structure | Mean paired difference (SD) | p value | Mean paired difference (SD) | p value | Mean paired difference (SD) | p value |
| Normal tissues | ||||||
| Right lung | ||||||
| Mean dose Gy) | 2.46 (1.34) | 0.0003 | –0.18 (0.66) | 0.4119 | –0.28 (0.62) | 0.1886 |
| V2 (%) | 64.98 (16.24) | <0.0001 | –7.03 (13.05) | 0.1226 | –7.94 (13.16) | 0.0889 |
| V5 (%) | 9.19 (21.57) | 0.2107 | –1.63 (5.13) | 0.3408 | –1.75 (5.09) | 0.3055 |
| V10 (%) | 0.94 (4.18) | 0.4947 | –0.35 (0.96) | 0.2855 | –0.35 (0.96) | 0.2753 |
| Right breast/chest wall | ||||||
| Mean dose Gy) | 2.03 (0.83) | <0.0001 | 0.36 (0.34) | 0.0083 | 0.20 (0.34) | 0.1013 |
| V2 (%) | 48.38 (24.54) | 0.0002 | 1.22 (7.40) | 0.6159 | –1.44 (8.29) | 0.5963 |
| V5 (%) | 9.79 (8.42) | 0.0051 | 1.51 (1.27) | 0.0046 | 1.08 (1.20) | 0.0197 |
| V10 (%) | 0.09 (0.87) | 0.7601 | 0.72 (0.76) | 0.0151 | 0.57 (0.69) | 0.0291 |
| Left lung | ||||||
| Mean dose Gy) | 3.95 (2.05) | 0.0002 | 1.69 (2.18) | 0.0361 | –0.22 (1.80) | 0.7049 |
| V2 (%) | 24.81 (9.52) | <0.0001 | –6.89 (8.22) | 0.0264 | –9.34 (8.51) | 0.0070 |
| V5 (%) | 39.44 (8.94) | <0.0001 | –4.84 (10.27) | 0.1704 | –7.84 (9.97) | 0.0348 |
| V10 (%) | 25.32 (13.05) | 0.0002 | –3.69 (9.68) | 0.2589 | –6.71 (9.36) | 0.0495 |
| V20 (%) | 4.97 (4.21) | 0.0047 | 1.33 (4.39) | 0.3617 | –1.87 (3.87) | 0.1602 |
| V30 (%) | –2.26 (4.32) | 0.1325 | 5.75 (4.80) | 0.0043 | 2.22 (4.00) | 0.1127 |
| V40 (%) | –2.49 (5.27) | 0.1694 | 9.64 (5.74) | 0.0005 | 5.30 (4.95) | 0.0081 |
| V50 (%) | –1.17 (2.13) | 0.1156 | 5.99 (3.26) | 0.0003 | 1.76 (1.58) | 0.0064 |
| Heart | ||||||
| Mean dose Gy) | 4.58 (1.39) | <0.0001 | 1.51 (1.74) | 0.0225 | –0.69 (1.20) | 0.1029 |
| V2 (%) | 48.47 (31.77) | 0.0009 | –3.72 (26.12) | 0.6636 | –13.84 (25.08) | 0.1149 |
| V5 (%) | 70.02 (15.05) | <0.0001 | 2.32 (13.47) | 0.5998 | –6.75 (13.33) | 0.1439 |
| V10 (%) | 9.03 (7.73) | 0.0050 | 4.65 (4.79) | 0.0134 | –1.90 (3.37) | 0.1080 |
| V20 (%) | –0.09 (0.83) | 0.7481 | 4.43 (3.06) | 0.0013 | –0.37 (0.81) | 0.1856 |
| V30 (%) | –0.05 (0.17) | 0.4215 | 3.29 (2.36) | 0.0017 | –0.05 (0.17) | 0.3896 |
| LAD artery | ||||||
| Mean dose Gy) | 5.79 (2.20) | <0.0001 | 15.14 (8.34) | 0.0003 | 0.56 (2.61) | 0.5152 |
| Max dose to 1% volume (Gy) | 9.51 (6.65) | 0.0014 | 36.79 (14.31) | <0.0001 | 4.41 (6.81) | 0.0709 |
| Targets | ||||||
| Whole breast PTV | ||||||
| Mean dose (Gy) | 0.13 (1.47) | 0.7941 | 2.70 (1.92) | 0.0016 | 0.30 (1.36) | 0.5017 |
| D95 Gy) | 4.39 (5.10) | 0.0236 | 5.55 (5.43) | 0.0102 | –0.89 (5.28) | 0.6078 |
| Max dose to 1% volume (Gy) | –1.81 (1.51) | 0.0043 | 2.80 (1.75) | 0.0007 | 1.93 (1.46) | 0.0023 |
| V47.5 (%) | 2.27 (2.50) | 0.0183 | 4.08 (3.48) | 0.0049 | –0.58 (2.95) | 0.5514 |
| Internal Mammary region | ||||||
| Mean dose (Gy) | 4.17 (7.98) | 0.1332 | –3.45 (8.59) | 0.2360 | –5.52 (7.80) | 0.0520 |
| D95 (Gy) | 4.22 (10.70) | 0.2436 | –7.73 (13.05) | 0.0938 | –13.15 (11.26) | 0.0050 |
| Max dose to 1% volume (Gy) | 2.12 (5.41) | 0.2461 | –3.78 (7.24) | 0.1333 | –5.01 (6.82) | 0.0453 |
| V47.5 (%) | 15.28 (26.54) | 0.1019 | –7.04 (20.96) | 0.3158 | –18.20 (17.63) | 0.0098 |
| Supraclavicular region | ||||||
| Mean dose (Gy) | 0.57 (1.54) | 0.2717 | –0.67 (2.81) | 0.4692 | –0.65 (2.36) | 0.4064 |
| D95 (Gy) | –0.06 (3.17) | 0.9543 | 0.45 (4.42) | 0.7561 | –0.68 (3.75) | 0.5796 |
| Max dose to 1% volume (Gy) | –0.03 (3.14) | 0.9793 | –0.73 (3.47) | 0.5252 | –2.31 (3.54) | 0.0698 |
| Infraclavicular region | ||||||
| Mean dose (Gy) | 0.59 (1.88) | 0.3494 | –0.40 (2.57) | 0.6325 | –2.14 (2.72) | 0.0343 |
| D95 (Gy) | 0.11 (2.86) | 0.9092 | –3.02 (5.58) | 0.1210 | –5.29 (4.92) | 0.0078 |
| Max dose to 1% volume (Gy) | 0.19 (2.12) | 0.7820 | 0.19 (2.28) | 0.7908 | –1.83 (2.84) | 0.0719 |
| Boost Volume | ||||||
| Mean dose Gy) | –0.87 (1.75) | 0.1521 | 2.58 (2.01) | 0.0029 | 1.64 (2.09) | 0.0354 |
| D95 (Gy) | –0.86 (2.12) | 0.2328 | –0.30 (6.56) | 0.8874 | 0.93 (2.53) | 0.2752 |
| Max dose to 1% volume (Gy) | –1.31 (1.68) | 0.0352 | 2.87 (1.86) | 0.0009 | 1.90 (1.63) | 0.0050 |
| V47.5 (%) | –0.13 (1.34) | 0.7615 | 0.30 (0.97) | 0.3540 | 0.37 (0.88) | 0.2134 |
Abbreviations: V2 = percentage of the volume receiving 2 Gy or more, e.g., V5 is the percentage of the volume receiving 5 Gy or more, etc.; D95 = lowest dose received by at least 95% of the volume; SD = standard deviation.
When the segmental technique was compared to the tangential beamlet technique, mean heart dose was higher with the segmental technique, as were the volumes of heart receiving 10, 20, and 30 Gy. The mean dose to the LAD artery was substantially higher with the segmental technique (20.6 Gy with the segmental technique versus 5.4 Gy with the tangential beamlet technique; mean paired difference, 15.1 Gy, p < 0.001), as was the maximum dose to the LAD artery. The mean dose to the contralateral breast was slightly higher with the segmental technique, as were the volumes receiving 5 and 10 Gy. The left lung mean dose was higher with the segmental than with the tangential beamlet technique, as were the volumes receiving 30, 40, and 50 Gy (mean V30 was 25.8% with the segmental technique and 20.0% with the tangential beamlet technique; mean paired difference, 5.8%; p = 0.004). Coverage of nodal targets was similar. Greater hot spots in the whole breast and boost volumes were seen with the segmental technique. Coverage of the whole breast volume was better with the segmental technique; the mean D95 was 45.9 Gy with the tangential beamlet technique compared with 51.4 Gy with the segmental technique.
Compared to the tangential beamlet technique, the 9-field technique was found to deliver higher mean doses to the contralateral breast (by a mean of 2.0 Gy), and substantially higher volumes received 2 and 5 Gy (mean V2 of 58.9% with the 9-field technique versus 10.6% with tangential beamlet technique; mean paired difference, 48.4; p < 0.001). The 9-field technique delivered higher mean dose to the right lung (by a mean of 2.5 Gy), and a substantially higher volume of the right lung received 2 Gy with the 9-field technique (mean V2 of 75.3% with the 9-field versus 10.3% with the tangential beamlet technique; mean paired difference 65.0; p < 0.001). The 9-field technique delivered higher mean dose to the left lung, and higher volumes of the left lung received 2, 5, 10, and 20 Gy with the 9-field technique. Mean heart dose was also higher with the 9-field technique, as were the volumes receiving 2, 5, and 10 Gy. The mean dose to the LAD artery was higher with the 9-field technique, as was the maximum dose received by 1% of the LAD artery volume.
Slightly greater hot spots were found in the whole breast and boost volumes with the tangential beamlet technique compared with the 9-field technique. Coverage of the whole-breast volume was better with the 9-field technique, with mean D95 of 50.2 Gy with the 9-field technique and 45.9 Gy with the tangential beamlet technique.
When the segmental blocked technique was compared to the tangential beamlet technique, normal tissue doses were generally similar. The segmental blocked technique resulted in slightly higher volumes of the contralateral breast receiving 5 and 10 Gy (mean paired difference in V5 = 1.1%; p = 0.02) and higher volumes of ipsilateral lung receiving 40 and 50 Gy (mean paired difference in V40 = 5.3%; p = 0.008) but lower volumes of the ipsilateral lung receiving 2, 5, and 10 Gy (mean paired difference in V2 = 9.3 Gy; p = 0.007). Nodal coverage was better with the tangential beamlet technique than the segmental blocked technique, with mean D95 to the internal mammary region of 43.8 Gy vs. 30.6 Gy (mean paired difference of 13.2 Gy; p = 0.005).
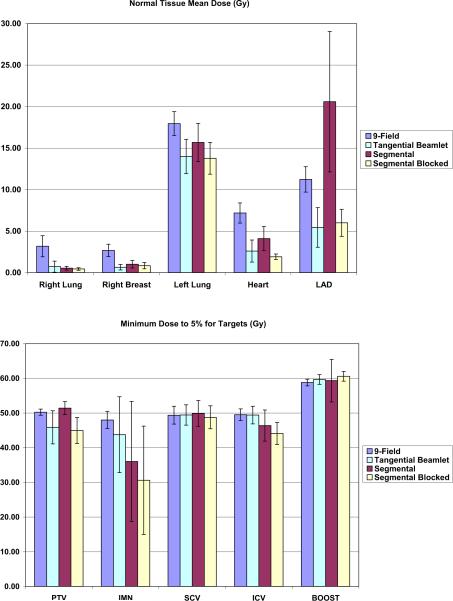
Figure 3 shows the mean doses to critical normal tissues and minimum doses to targets achieved with each of the four techniques.
Fig. 3.
Mean doses to critical normal tissues and minimum doses to targets achieved with each of the four techniques. SCV = supraclavicular planning target volume; ICV = infraclavicular planning target volume; IMN = internal mammary planning target volume, PTV = planning target volume; LAD = left anterior descending coronary artery.
DISCUSSION
In this study, we compared four techniques for intensity-modulated treatment of the breast and regional lymph nodes in the setting of a deep inspiration breath hold. As expected, our findings demonstrated tradeoffs between the four techniques. The 9-field technique achieves excellent target coverage but delivers considerable dose to contralateral normal tissues. The segmental technique delivers excessive doses to the heart, and the segmental blocked technique must compromise nodal coverage to reduce cardiac dose. The tangential beamlet technique is the best compromise of target coverage and sparing of normal tissues, as it achieves substantially lower doses to critical normal tissues than the 9-field and the segmental techniques, with similar nodal coverage and only slightly decreased minimum dose coverage of the whole-breast PTV.
This work builds upon previous work at our institution and elsewhere. Over a decade ago, Stanford researchers proposed inverse-planned IMRT using nine equispaced beams as a potentially useful technique by which to treat the breast and regional lymph nodes (25). Investigators at the University of Wisconsin subsequently reported a treatment planning study in one patient that demonstrated improvements in dose to the breast, internal mammary nodes, left lung, and heart with inverse-planned IMRT with tomotherapy compared to standard tangents matched to a mixed beam internal mammary field (26). German investigators showed improved target coverage and reduction in the high-dose exposure of heart and lung but at the expense of higher low-dose exposure to normal tissues with a 12-field IMRT technique (27). Dutch investigators found that inverse-planned intensity-modulated tangential photon fields led to the best coverage of the breast and internal mammary nodes while resulting in lower normal tissue complication probabilities than a wide split tangential plan (28).
Investigators at Beaumont Hospital explored the use of tangential beams divided into multiple relatively large segments and an uncompensated direct supraclavicular beam in the setting of treatment to the breast and regional nodes, as well as the use of a deep inspiration breath hold (24). They found that compared with shallow tangents matched with electrons, deep tangents blocked below the superior internal mammary nodes in conjunction with deep inspiration breath hold led to reduction of heart dose in most patients, with comparable lung toxicity parameters, but at the expense of increased dose to the opposite breast. They again found improved dose homogeneity with their IMRT technique, along with slight reduction in heart dose, and theirs was the technique that led to the heart-blocked segmental technique in this study. Investigators at Memorial Sloan-Kettering demonstrated that a simplified IMRT algorithm applied to the tangential beams was feasible in the context of a three-field breast treatment with a single isocenter, intended to allow treatment of the supraclavicular and/or axillary nodes in addition to the breast. In that study, the use of the simplified IMRT technique resulted in improved dose distributions within the breast target, as well as reduced dose to the contra-lateral breast and reduced hot spots in the ipsilateral lung (29). More recently, Dogan and colleagues (30) reported experience with a variety of IMRT techniques, concluding that two- and four-field IMRT plans provided the best balance between target coverage and normal tissue sparing. However, their discussion of the impact of 9-field IMRT treatment on contralateral tissues was limited to an analysis of D2, rather than an assessment of volumes exposed to very low doses, which may be particularly clinically relevant when considering the possibility of carcinogenesis (31).
Studies of IMRT for breast cancer at our institution began by considering a 9-field technique for treatment of the chest wall and regional nodes. As noted in the study reporting our treatment planning experience with this technique (19), doses to contralateral structures made it necessary to conduct further treatment planning studies, in which cost functions were adjusted and ultimately alternative beam arrangements were explored, before utilizing beamlet IMRT in the clinical setting. In light of the further treatment planning studies we have conducted, including the results reported here, we conclude that although the 9-field technique was a reasonable initial approach in the development of a clinically applicable technique for the IMRT treatment of the breast or chest wall and regional nodes, it is now of historical importance only, as it would result in increased radiation exposure to normal tissues, including low doses that could predispose to increased second malignancy risk. In light of the findings of the Oxford meta-analysis revealing a significant increase in contralateral breast and lung cancer in patients receiving radio-therapy for the treatment of breast cancer in randomized trials (1), we feel that it is particularly critical that new radiation techniques are carefully designed to minimize even low-dose exposure of these structures. Thus, we do not believe that equispaced beam techniques such as the 9-field technique merit further study. Rather, a technique such as the tangential beamlet technique with electrons when necessary, as described here, can achieve reasonable target coverage with significant improvements in the sparing of normal tissues.
CONCLUSIONS
IMRT utilization is increasing, and the technology is now being applied in settings in which its benefits have not been clearly established. Great heterogeneity exists in what is defined as “breast IMRT.” Segmental breast IMRT delivered with a traditional field arrangement and intended to improve dose homogeneity within the breast has been evaluated in prospective trials, and improvements in skin and soft tissue toxicity have been demonstrated. More sophisticated beamlet techniques incorporating dose constraints to other normal tissues have not been evaluated prospectively, nor has any effect on clinical cardiac or pulmonary outcomes been shown from the use of any IMRT technique. Ultimately, decisions about the use of each type of IMRT should be based upon rigorous analysis of patient outcomes through prospective trials.
While the dosimetric results presented in this study are promising, further study in the clinical setting will be necessary before the widespread adoption of beamlet IMRT for the treatment of the breast and regional nodes. The primary contribution of the present study is the identification of a technique that appears suitable for such necessary study in the clinical setting.
Acknowledgments
This work was supported in part by grants from the Breast Cancer Research Foundation and National Institutes of Health (R01-CA102435) to Dr. Pierce and from the American Cancer Society (MRSG-09-145-01) to Dr. Jagsi.
Footnotes
Reprint requests to: Reshma Jagsi, M.D., D.Phil., 1500 East Medical Center Drive, Ann Arbor, MI 48109. Tel: (734)936-7810; Fax: (734)763-7370; rjagsi@med.umich.edu
Presented at the 51st Annual Meeting of the American Society for Radiation Oncology, Chicago, IL, Oct. 31-Nov. 4, 2009.
Conflicts of interest: none.
REFERENCES
- 1.Early Breast Cancer Trialists’ Collaborative Group Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 2.Pignol J, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26(13) doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 3.Donovan E, Bleakley N, Denholm E, et al. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol. 2007;82(3):254–264. doi: 10.1016/j.radonc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Harsolia A, Kestin L, Grills I, et al. Intensity-modulated radio-therapy results in significant decrease in clinical toxicities compared with conventional wedge-based breast radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68(5):1375–1380. doi: 10.1016/j.ijrobp.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 5.Evans PM, Donovan EM, Partridge M, et al. The delivery of intensity modulated radiotherapy to the breast using multiple static fields. Radiother Oncol. 2000;57:79–89. doi: 10.1016/s0167-8140(00)00263-2. [DOI] [PubMed] [Google Scholar]
- 6.Donovan EM, Bleackley NJ, Evans PM, et al. Dose-position and dose–volume histogram analysis of standard wedged and intensity modulated treatments in breast radiotherapy. Br J Radiol. 2002;75:967–973. doi: 10.1259/bjr.75.900.750967. [DOI] [PubMed] [Google Scholar]
- 7.Kestin LL, Sharpe MB, Frazier RC, et al. Intensity modulation to improve dose uniformity with tangential breast radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48(5):1559–1568. doi: 10.1016/s0360-3016(00)01396-1. [DOI] [PubMed] [Google Scholar]
- 8.Vicini FA, Sharpe M, Kestin L, et al. Optimizing breast cancer treatment efficacy with IMRT. Int J Radiat Oncol Biol Phys. 2002;54(5):1336–1344. doi: 10.1016/s0360-3016(02)03746-x. [DOI] [PubMed] [Google Scholar]
- 9.Cuzick J, Stewart H, Rutqvist L, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12(3):447–453. doi: 10.1200/JCO.1994.12.3.447. [DOI] [PubMed] [Google Scholar]
- 10.Harris EER, Correa C, Hwang W-T, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24(25):4100–4106. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 11.Jagsi R, Griffith KA, Koelling T, et al. Rates of myocardial infarction and coronary artery disease and risk factors in patients treated with radiation therapy for early-stagebreastcancer. Cancer. 2007;109(4):650–657. doi: 10.1002/cncr.22452. [DOI] [PubMed] [Google Scholar]
- 12.Marks LB, Yu X, Prosnitz RG, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63(1):214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Zambetti M, Moliterni A, Materazzo C, et al. Long-term cardiac sequelae in operable breast cancer patients given adjuvant chemotherapy with or without doxorubicin and breast irradiation. J Clin Oncol. 2001;19(1):37–43. doi: 10.1200/JCO.2001.19.1.37. [DOI] [PubMed] [Google Scholar]
- 14.Sparano JA. Cardiac toxicity of trastuzumab. Semin Oncol. 2001;28(Suppl 3):20–27. doi: 10.1016/s0093-7754(01)90189-7. [DOI] [PubMed] [Google Scholar]
- 15.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med. 1997;337(14):949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 16.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337(14):956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 17.Musat E, Poortmans P, Bartelink H, et al. Patient population comparison between EORTC randomized trials. Int J Radiat Oncol Biol Phys. 2005;63(Suppl 1):S239–SS40. [Google Scholar]
- 18.Pritchard K, Whelan T. Clinical trial update: NCIC. Breast Cancer Res. 2005;7(2):48–51. doi: 10.1186/bcr979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krueger EA, Fraass BA, McShan DL, et al. Potential gains for irradiation of chest wall and regional nodes with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56(4):1023. doi: 10.1016/s0360-3016(03)00183-4. [DOI] [PubMed] [Google Scholar]
- 20.Lohr F, El-Haddad M, Dobler B, et al. Potential effect of robust and simple IMRT approach for left-sided breast cancer on cardiac mortality. Int J Radiat Oncol Biol Phys. 2009;74(1):73–80. doi: 10.1016/j.ijrobp.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Moran JM, Balter JM, Ben-David MA, et al. The displacement and reproducibility of the breast and nodal targets under active breathing control. Int J Radiat Oncol Biol Phys. 2007;68:541–546. doi: 10.1016/j.ijrobp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madu CN, Quint DJ, Normolle DP, et al. Definition of the supraclavicular and infraclavicular nodes. Radiology. 2001;221(2):333–339. doi: 10.1148/radiol.2212010247. [DOI] [PubMed] [Google Scholar]
- 23.Kessler ML, McShan DL, Epelman MA, et al. Costlets: A generalized approach to cost functions for automated optimization of IMRT treatment plans. Optimization and Engineering. 2005;6:421–448. [Google Scholar]
- 24.Remouchamps VM, Vicini FA, Sharpe MB, et al. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys. 2003;55:392–406. doi: 10.1016/s0360-3016(02)04143-3. [DOI] [PubMed] [Google Scholar]
- 25.Smitt MC, Li SD, Shostak CA, et al. Breast-conserving radiation therapy: potential of inverse planning with intensity modulation. Radiology. 1997;203:871–876. doi: 10.1148/radiology.203.3.9169719. [DOI] [PubMed] [Google Scholar]
- 26.Reckwerdt PJ, Olivera G, Shepard DM, et al. Case studies in tomotherapy optimization: Breast, prostate, mesothelioma, and nasopharyngeal treatments.. Proceedings of the XV International Conference on Computers in Radiation Therapy; Heidelberg, Germany. 2000. [Google Scholar]
- 27.Thilmann C, Zabel A, Nill S, et al. Intensity-modulated radio-therapy of the female breast. Med Dosim. 2002;27:79–90. doi: 10.1016/s0958-3947(02)00089-4. [DOI] [PubMed] [Google Scholar]
- 28.Cho BCJ, Hurkmans CW, Damen EMF, et al. Intensity modulated versus non-intensity modulated radiotherapy in the treatment of the left breast and upper internal mammary lymph node chain. Radiother Oncol. 2002;62:127–136. doi: 10.1016/s0167-8140(01)00472-8. [DOI] [PubMed] [Google Scholar]
- 29.Chui CS, Hong L, McCormick B. Intensity-modulated radio-therapy technique for three-field breast treatment. Int J Radiat Oncol Biol Phys. 2005;62:1217–1223. doi: 10.1016/j.ijrobp.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 30.Dogan N, Cuttino L, Lloyd R, et al. Optimized dose coverage of regional lymph nodes in breast cancer: the role of IMRT. Int J Radiat Oncol Biol Phys. 2007;68:1238–1250. doi: 10.1016/j.ijrobp.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 31.Stovall M, Smith SA, Langholz BM, et al. Dose to the contra-lateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int J Radiat Oncol Biol Phys. 2008;72(4):1021–1030. doi: 10.1016/j.ijrobp.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]