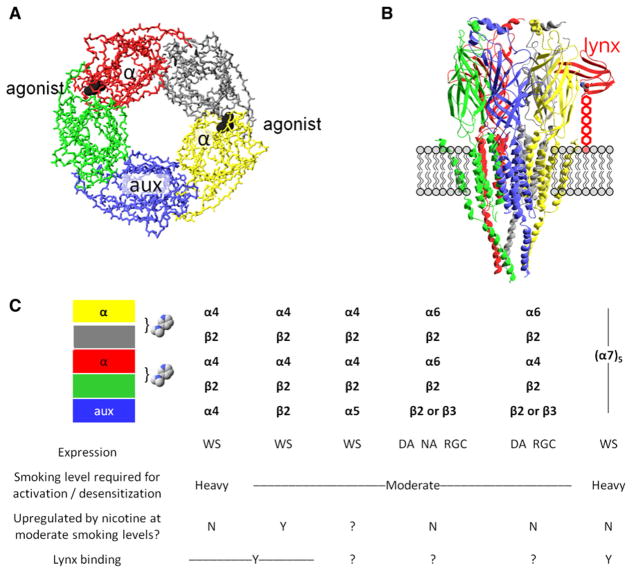
Figure 1. Major Characteristics of Some nAChRs.
(A) A diagram of the symmetric or pseudosymmetric pentameric extracellular binding region, modeled by the acetylcholine receptor binding protein AChBP. The eyepoint is the cytosol; the side chains and transmembrane domains do not appear. The exemplar agonist (nicotine) is represented in black; two agonist binding sites form at the interface between subunits. The open state of the ion channel is more likely to occur when agonist molecules bind at both interfaces than at a single interface. An α subunit (red and yellow) always participates in the binding interface; the other participants are either α subunits (in α7 homopentameric nAChRs) or non-α subunits (in heteropentameric nAChRs such as α4β2*); (see the table in C). The auxiliary subunit (aux, in blue) does not participate in an agonist binding site.
(B) Depiction of a nAChR molecule in the membrane. The eyepoint is a neighboring nAChR. The receptor is Unwin’s model for the Torpedo electric organ muscle-type AChR (Unwin, 2005). The model depicts the full extracellular region (mostly β sheets), which strongly resembles the AChBP structure shown in (A). Ribbons depict the structural elements, whereas neither backbone nor side-chain atoms appear. The model includes the full transmembrane region (mostly α-helical) and only part of the intracellular domains. The schematic also imagines a lynx molecule (red) bound at an α/non-α interface, positioned as in structures of snake α-toxins bound to AChBP (Hansen et al., 2005) or to the muscle nAChR (Dellisanti et al., 2007). Lynx binding, as independently proposed in a recent study (Lyukmanova et al., 2011), occurs at the agonist site shown in (A). The lynx molecule, unlike toxins, is tethered to the membrane by a GPI linkage, here stretched to nearly its full extent and depicted as five hexagons.
(C) Some major nAChR subtypes found in brain. Each column represents the composition of a single pentameric receptor. The table shows our best present knowledge about the properties of detailed stoichiometries. The colored boxes correspond to the subunits of (A) and (B). The bracket and the nicotine molecules show the agonist-binding interfaces between individual subunits. Expression of each receptor subtype is wide-spread (WS), or restricted in the case of α6* nAChRs, confined largely to dopaminergic neurons (DA), noradrenergic neurons (NA), or retinal ganglion cells (RGC).