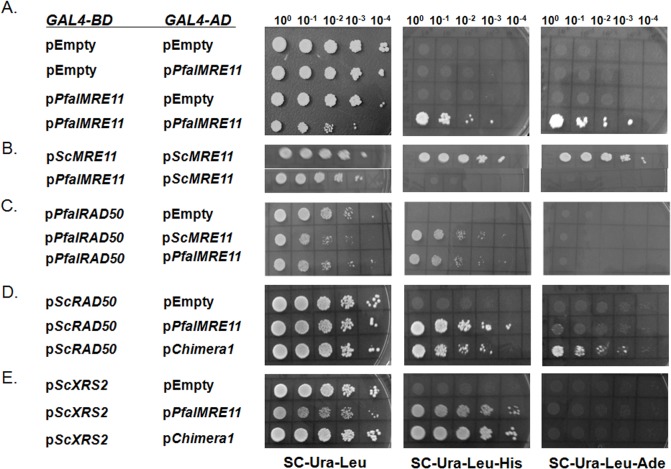
Fig 6. PfalMre11 is capable of forming MRX complex in yeast.
(A) A DNA fragment corresponding to the full length PfalMRE11 ORF was fused to the GAL4 activation domain (GAL4-AD) in pGADC1 as well as to the GAL 4 DNA binding domain (GAL-BD) in pGBDUC1. Two hybrid interactions were tested with yeast strain PJ694A, which carries ADE2 and HIS3 genes as reporters. Starting with the same OD (1 OD/ml), five fold serial dilutions were prepared and spotted on media lacking uracil and leucine (SC-Ura-Leu) as a control, as well as media lacking uracil, leucine and histidine (SC-Ura-Leu-His) or media lacking uracil, leucine and adenine (SC-Ura-Leu-Ade) to test for protein-protein interactions. (B) ScMRE11 or PfalMRE11 were cloned into pGBDUC1 to produce fusion protein with GAL4 DNA binding domain. Interactions with ScMRE11 were scored. (C) PfalRAD50 were cloned into pGBDUC1 to produce fusion protein with GAL4 DNA binding domain. Interactions with ScMRE11 or PfalMRE11 were scored. (D) ScRAD50 was cloned into pGBDUC1 to produce a fusion protein with a GAL4 DNA binding domain. Interactions with PfalMRE11 or Chimera 1 were scored. (E) ScXRS2 was fused to the DNA binding domain in pGBDUC1 and tested for interaction with PfalMRE11 or Chimera 1.