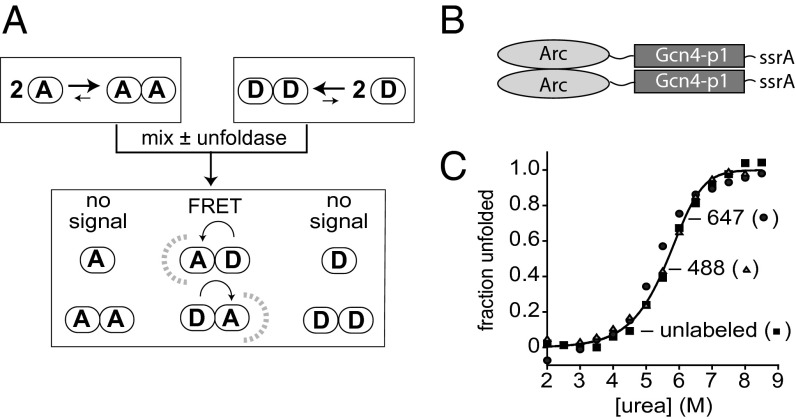
Fig. 1.
Subunit-exchange assay and Arc-Gcn4-ssrA dimers. (A) Following mixing of dimers labeled with donor or acceptor fluorophores, spontaneous or enzyme-induced unfolding/dissociation allows subunit mixing and FRET. Assuming equal stabilities, donor, mixed, and acceptor dimers should be present in a 1:2:1 ratio at equilibrium. (B) Arc-Gcn4-ssrA contains the R23C Arc protein, a 10-residue linker, the Gcn4p1 peptide, and an st11-ssrA tag for purification and targeting to the ClpX and ClpA unfoldases. (C) Unlabeled Arc-Gcn4-ssrA dimers (squares; 2.5 µM), Alexa-488 labeled Arc-Gcn4-ssrA dimers (triangles; 2.5 µM), or Alexa-647 labeled Arc-Gcn4-ssrA dimers (circles; 2.5 µM) were incubated with different concentrations of urea for 20 h at room temperature and the fraction unfolded was calculated from the circular-dichroism ellipticity at 222 nm, assuming flat native and unfolded baselines. The solid line is a global fit to a N2 ⇔ 2U model with a ∆Gu of 15.9 ± 0.5 kcal/mol in the absence of denaturant (corresponding to Kdimer ∼ 10−12 M) and an m value of 1.55 ± 0.15 kcal/mol⋅M.