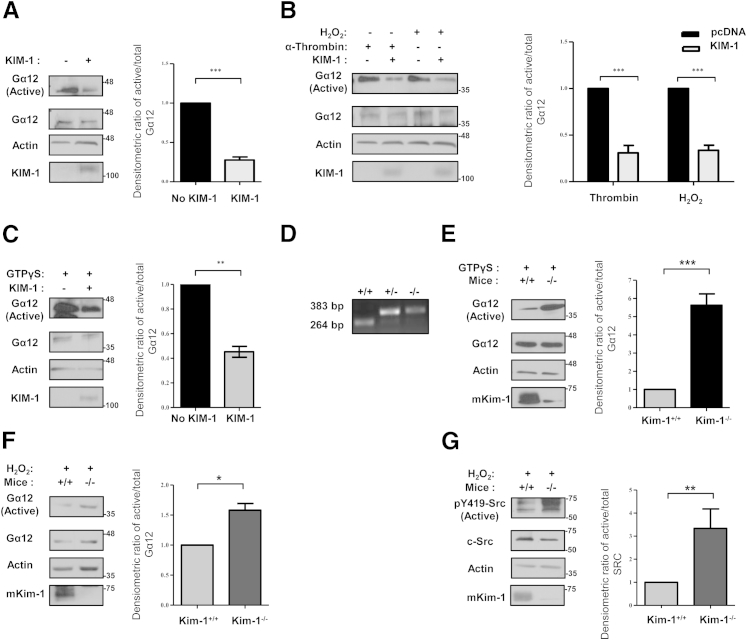
Figure 2.
KIM-1 inhibits cellular Gα12 activation by blocking GTP-binding. HEK-293 cells were transfected with either control vector (pcDNA) or KIM-1 plasmid. Cells were left untreated (A), stimulated with either 2 U/mL α-thrombin or 5 mmol/L H2O2 for 30 minutes (B), or treated with GTPγS for 15 minutes (C). D: Confirmation of genotype by using genomic DNA of wild-type (+/+), heterozygous (+/−), and homozygous Kim-1 knockout (−/−) C57BL/6 mice by PCR. TECs isolated from wild-type (Kim-1+/+) and Kim-1–deficient (Kim-1−/−) mice were stimulated with GTPγS (E) or 5 mmol/L H2O2 for 30 minutes (F). Samples in A–C, E, and F were subjected to GST-TPR pull-down assay to measure the amount of Gα12 activation. Lysates (total) and pull-down (active) samples were analyzed by SDS-PAGE and Western analysis with antibodies against Gα12, human KIM-1 or mKim-1, and actin where indicated. G: Activated and total Src were detected in total cell lysates from TECs stimulation with H2O2 by Western blot analysis by using anti–p-Src (Py419-Src) phosphospecific antibody and anti–c-Src antibody, respectively. Densitometric analysis of the ratio of active to total Gα12 (or pY419-Src to c-Src) relative to non–KIM-1-expressing cells or wild-type TECs (+/+) is shown as a representation the experiments. Data are expressed as means ± SEM. n = 3 independent experiments. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Gα12, G protein α-12; GST-TPR, glutathione S-transferase–fused tetratricopeptide repeat domain of Ser/Thr protein phosphatase type 5; GTPγS, nonhydrolyzable GTP analog; HEK, human embryonic kidney; KIM-1, kidney injury molecule-1; mKim-1, mouse kidney injury molecule-1; TEC, tubular epithelial cell.