FIGURE 2.
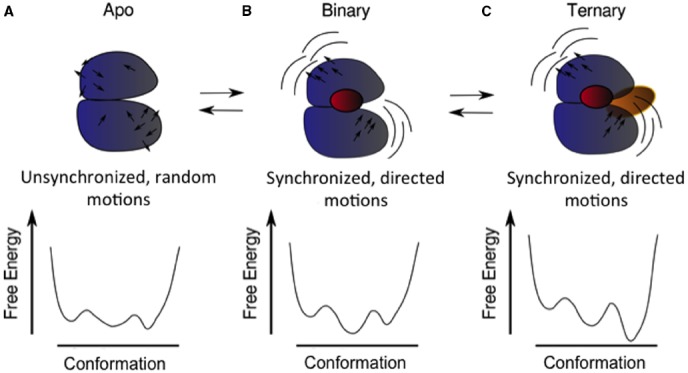
Schematic of structural and dynamic coupling between different functional sites in a protein and how this coupling could modulate the energy landscape. The apo or ligand-free protein (A) samples three conformations in its native basin and displays unsynchronized and undirected motions (black arrows). Upon binding of the first ligand (B), a particular conformation is selected (the minimum in the middle of the landscape), and also the motions of the two main lobules of the protein become synchronized (notice the similar direction of the arrows in each domain). These motions favor the binding of the second ligand (C), selecting another of the possible conformations.
