Figure 1.
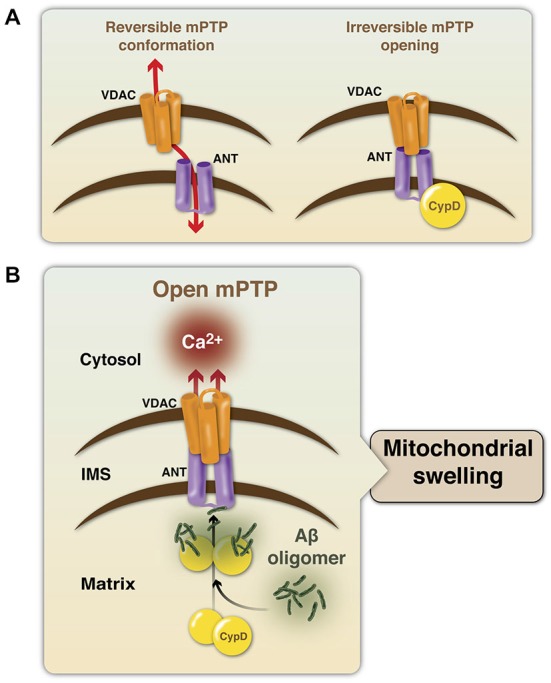
Modulation of the mitochondrial permeability transition pore structure. (A) The mPTP is mainly formed by the voltage-dependent calcium channel (VDAC, in orange), adenine nucleotide translocase (ANT, in purple) and cyclophilin D (CypD, in yellow). Under a physiological state, the mPTP presents a reversible conformation and CypD is not part of the protein complex and mainly resides into the mitochondrial matrix (MM). An interaction of the components, triggered by an apoptotic stimulus, induces the formation and opening of the mPTP, generating an irreversible response, that allows the entry of water and solutes into the MM. (B) In the context of AD, Aβ oligomers induce the translocation of CypD from the MM to the IMM to facilitate their interaction with ANT, and therefore the formation and opening of the mPTP. This final step induces uncontrolled release of calcium and mitochondrial swelling.
