Abstract
Children in low income countries are the least touched by recent successes in the diagnosis and treatment of HIV/AIDS globally. Early treatment is essential for a child’s longer and higher quality of life; however, by 2011 only a small proportion of HIV-seropositive children in low- and middle-income (LMIC) countries were receiving treatment, in part because of persisting low rates of diagnosis. This study of the prevalence and characteristics of children tested for HIV was embedded in the Coping with HIV/AIDS in Tanzania (CHAT) study in which HIV-seropositive, and HIV-seronegative adults, and adults with unknown HIV status were asked about HIV testing for their children. Data were gathered from November 2009 to August 2010 during the scale up of Prevention of Mother to Child Transmission and Early Infant Diagnosis programs in the region. Reports on 1,776 children indicate that 31.7% of all children were reported to have been tested, including only 42.9% of children with an HIV-seropositive caregiver. In general, children more likely to be HIV tested were a biological child of study participants, younger, of widowed adults, in urban areas, and of HIV-seropositive parents/caregivers. Children belonging to the two indigenous tribes, Chagga and Pare, were more likely to be tested than those from other tribes. Rates of testing among children less than two years old were low, even for the HIV-seropositive caregiver group. The persistence of low testing rates is discussed in terms of the accessibility and acceptability of child testing in resource poor settings.
Keywords: AIDS, HIV testing, infants, children, Tanzania
Introduction
Despite progress in the battles to prevent and treat HIV/AIDS globally, children in LMIC are the least touched by the success. Early identification and treatment of HIV disease is essential for a longer and higher quality of life. Infants with perinatal infection are the most likely to benefit from treatment without which 50% will die or progress to AIDS defining symptoms by age one (De Cock, Fowler, Mercier et al., 2000; Dabis & Edpini, 2002; Walker, Schwartlander, & Bryce, 2002; Berk, Falkovitz-Halpern, Hill, et al., 2005; Violari, Cotto, Gibb et al., 2008.). Eighty percent of untreated children will die by age five (Newell, Brahmbhatt & Ghys, 2004; Newell, Coovadia, Cortina-Borja et al. 2004). Only a small proportion of children in LMIC receive treatment (28% relative to 58% for adults) (UNAIDS, 2012), in part because of persisting low rates of HIV testing and diagnosis (e.g., Kellerman & Essajee, 2010.)
This report draws from a longitudinal study of adults in an area in East Africa with high risk for HIV/AIDS. In this study, adult participants were asked whether or not their child(ren) had been tested for HIV. The data were gathered during the roll out of early infant diagnosis (EID) with dried blood spot testing (DBS) for infants and a large scaling up of PMTCT programs in the area.
Methods
This study of the frequency and characteristics of children being tested for HIV was conducted in the Kilimanjaro Region in Northern Tanzania, an appropriate setting for studying HIV testing since it is located along a major East African transit route with relatively high HIV/AIDS prevalence and multiple testing sites within a well-circumscribed area. These data were gathered within the NIH-funded Coping with HIV/AIDS in Tanzania (CHAT; NIH #5R01MH078756-05; K. Whetten, PI). Study activities were approved by theInstitutional Review Boards of Kilimanjaro Christian Medical Center (KCMC) and the Duke University Health System, and written informed consent was obtained from all participants. The sampling strategy and other findings from the CHAT study are published elsewhere (refs.)
Study Population
In 2009, 1,197 adults were recruited for the CHAT study in five cohorts from three districts of the Kilimanjaro region: (1) patients with established HIV infection receiving care at two regional referral hospitals (KCMC, n=228 and Mawenzi Hospital n=271); (3) HIV+ individuals newly diagnosed at voluntary counseling and testing (VCT) sites (n=267); (4) individuals testing HIV-seronegative at VCT sites (n=182); and (5) a random sample of adults from the surrounding community (n=249). The data in this report were collected in round 3, the second round of follow-up interviews, conducted between November 2009 and August 2010.
Procedures
Participants completed in-person one-hour interviews every six months with trained local interviewers not previously known to the participants. Interviews were conducted in Kiswahili, with instruments translated and back-translated to English to confirm the validity of the translation. Adult characteristics selected a priori as possible predictors of child testing included age, gender, marital status, educational attainment, tribe, urban versus rural residence, and whether the child was born after or in the year the caregiver was diagnosed with HIV (HIV seropositive group only). Child characteristics included age, gender, and whether the child was the biological child of the study participant. Neither the age at which the child was tested for HIV nor test results were asked.
Statistical Methods
Distributions for study variables were tested to ensure they met distributional assumptions for analyses. Means or proportions were compared among those children with (1) HIV-seropositive caregivers, (2) recently tested HIV seronegative caregivers, and (3) caregivers in the community sample, using the Student’s t test. Bivariate logistic regressions were used to explore associations between child testing and the hypothesized predictors. A multivariate logistic regression model was estimated to identify factors independently predictive of child testing. Statistical analyses were conducted using Stata software, version 11.2 (Ware, Kosinsky, & Dewey,2001).
Results
Description of participants
Table 1 describes the 973 (81.3% of the original sample) adult participants in round three interviews, 613 known to be HIV-seropositive, 135 recently tested HIV-seronegative; and 225 from a random community sample with unknown HIV status at recruitment. Nearly 81%, (80.7%; n= 785) reported that there were children in their households; and there were no group differences in this regard. Seventeen couples lived together with children; only the report of the female parent/caregiver was used to avoid double counting. In post hoc analyses, there were no differences in child testing reports when the male parents’/caregivers’ reports were used.
Table 1. Sample Characteristics.
| All adults | ||||
|---|---|---|---|---|
| All participants | HIV+ | VCT− | Community | |
|
|
||||
| Sample size | 973 | 613 | 135 | 225 |
| Reported children in household | ||||
| Yes | 80.7% (785) | 79.9% (490) | 77.0% (104) | 84.9% (191) |
| No | 19.3% (188) | 20.1% (123) | 23.0% (31) | 15.1% (34) |
|
| ||||
| Adults that have children in their households | ||||
| All participants | HIV+ | VCT− | Community | |
|
|
||||
| Number of children, mean (SD) | 2.3 (1.3) | 2.2 (1.2) | 2.4 (1.2) | 2.5 (1.3)** |
| Age (range: 18 - 64), mean (SD) | 39.4 (9.9) | 41.0 (8.6) | 32.8 (10.0) ** | 39.1 (11.5)* |
| Gender | ||||
| Male | 31.5% (246) | 26.4% (129) | 43.3% (45)** | 37.9% (72)** |
| Female | 68.5% (536) | 73.6% (359) | 56.7% (59)** | 62.1% (118)** |
| Marital status | ||||
| Married/cohabitating | 51.3% (401) | 42.6% (208) | 52.9% (55) | 72.6% (138)** |
| Never married | 12.8% (100) | 11.5% (56) | 22.1% (23)** | 11.1% (21) |
| Widowed | 20.5% (160) | 27.7% (135) | 8.7% (9)** | 8.4% (16)** |
| Divorced/separated | 15.5% (121) | 18.2% (89) | 16.3% (17) | 7.9% (15)** |
| Highest level of education | ||||
| None | 3.7% (29) | 5.1% (25) | 1.9% (2) | 1.1% (2)* |
| Primary (1 - 6 years) | 77.2% (603) | 76.8% (375) | 77.7% (80) | 77.9% (148) |
| Secondary or higher | 19.1% (149) | 18.0% (88) | 20.4% (21) | 21.1% (40) |
| Tribe | ||||
| Chagga | 64.8% (506) | 63.7% (311) | 54.4% (56) | 73.2% (139)* |
| Pare | 11.5% (90) | 13.1% (64) | 12.6% (13) | 6.8% (13)* |
| Other tribes | 23.7% (185) | 23.2% (113) | 33.0% (34) | 20.0% (38)** |
| Area of permanent residence | ||||
| Urban | 44.4% (347) | 41.0% (200) | 40.4% (42) | 55.3% (105) |
| Rural | 53.8% (421) | 56.8% (277) | 58.7% (61) | 43.7% (83) |
|
| ||||
| All Children | ||||
| All participants | Caregiver HIV+ | Caregiver VCT− | Community | |
|
|
||||
| Sample size at round 3 | 1776 | 1065 | 248 | 463 |
| Age (range: 0 - 17), mean (SD) | 9.5 (4.8) | 9.8 (4.8) | 9.0 (4.7)* | 9.0 (4.9)** |
| 0 - 4 years | 20.4% (362) | 18.5% (197) | 21.2% (52) | 24.4% (113)** |
| 5 - 12 years | 47.1% (835) | 46.3% (493) | 52.7% (129) | 46.0% (213) |
| 13 - 17 years | 32.5% (576) | 35.2% (375) | 26.1% (64)** | 29.6% (137)* |
| Gender | ||||
| Male | 49.0% (871) | 49.1% (523) | 50.0% (124) | 48.4% (224) |
| Female | 51.0% (905) | 50.9% (542) | 50.0% (124) | 51.6% (239) |
| Biological child of reporting caregiver | ||||
| Yes | 63.7% (1132) | 60.5% (644) | 62.9% (156) | 71.7% (332)** |
| No | 36.3% (644) | 39.5% (421) | 37.1% (92) | 28.3% (131)** |
| Reported to have been tested for HIV/AIDS | ||||
| Yes | 31.7% (563) | 42.9% (457) | 29.4% (73)** | 7.1% (33)** |
| No | 68.3% (1213) | 57.1% (608) | 70.6% (175)** | 92.9% (430)** |
Asterisks in the Caregiver VCT− and Community columns indicate a statistically significant difference relative to the caregiver HIV+ group:
p<0.05
p<0.01
The HIV-seropositive interviewees included significantly more females than the other groups and more widows than the HIV- group. In the community sample, significantly more participants report that they are married or cohabitating than in the HIV-seropositive group.
Adult caregivers reported on a total of 1,776 children (see Table 1). Children of HIV-seropositive caregivers were older than those in the HIV-seronegative and the community group. Nearly 64% of all children were the biological child, with adults in the community group reporting a higher percentage of their children as their biological offspring than in the HIV-seropositive group.
Only 31.7% (n= 563) of all children were reported by their caregivers to have been tested for HIV, including less than half (42.9%) of children with at least one HIV-seropositive caregiver.
Child Characteristics and Contextual Associations with Child HIV Testing
Bivariate results (not shown) indicate that if a caregiver was HIV-seropositive, younger children were more likely to be reported as having been tested for HIV (OR=0.96); whereas, for the community sample, older children were more likely to have been tested (OR= 1.10). Testing was not related to child or adult gender. Children living with biological parents who are HIV positive were more likely to be tested (OR= 4.23). There was a trend to suggest that among children living with HIV-seropositive caregivers, those born after or in the same year as the adult’s HIV diagnosis were more likely to be reported to have been HIV tested (OR =1.43; p<0.07).
Multivariate logistic regression models were estimated separately by caregivers’ HIV status, using the hypothesized predictors (see Table 2).
Table 2. Multivariable logistic regression in explanation of child being tested of HIV (N= 1,714).
| Caregiver HIV+ | Caregiver VCT− | Community | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
|
|
||||||
| Child age (continuous) | 0.93 | 0.89, 0.96 *** | 1.05 | 0.98, 1.12 | 1.13 | 1.04, 1.23 ** |
| Child gender (ref=male) | ||||||
| Female gender | 1.13 | 0.86, 1.49 | 1.49 | 0.85, 2.60 | 1.05 | 0.45, 2.46 |
| Caregiver gender (ref=male) | ||||||
| Female gender | 0.80 | 0.51, 1.25 | 0.85 | 0.34, 2.13 | 4.37 | 0.85, 22.38 |
| Caregiver marital status (ref=married/cohabitating) | ||||||
| Never married | 0.58 | 0.29, 1.19 | 0.41 | 0.12, 1.38 | 2.30 | 0.40, 13.30 |
| Widowed | 1.68 | 1.04, 2.71 * | 0.82 | 0.21, 3.28 | 1.49 | 0.31, 7.22 |
| Divorced/separated | 0.86 | 0.52, 1.40 | 0.79 | 0.25, 2.51 | 0.29 | 0.04, 2.11 |
| Caregiver highest level of education achieved | ||||||
| ≥ Secondary school | 0.85 | 0.50, 1.44 | 1.74 | 0.60, 5.04 | 0.21 | 0.03, 1.44 |
| Nature of caregiver-child relationship | ||||||
| Biological parent-child | 5.58 | 3.76, 8.29 *** | 1.50 | 0.58, 3.91 | 2.07 | 0.76, 5.63 |
| Caregiver tribe (ref=Chagga) | ||||||
| Pare | 1.28 | 0.70, 2.36 | 0.87 | 0.29, 2.68 | 2.72 | 0.72, 10.32 |
| Other tribes | 0.61 | 0.40, 0.93 * | 0.28 | 0.09, 0.89 * | 3.02 | 0.99, 9.24 |
| Area of permanent residence (ref=urban) | ||||||
| Rural/mixed | 0.67 | 0.46, 0.98 * | 0.69 | 0.31, 1.53 | 0.41 | 0.14, 1.21 |
| Child was born in/after the year caregiver was diagnosed with HIV | 1.11 | 0.68, 1.79 | NA | NA | ||
| Caregiver ever tested of HIV | NA | NA | 12.54 | 1.60, 98.08 * | ||
ref – reference group; *, ** and *** indicate statistical significance at the 0.05, 0.01, and 0.001 levels, respectively.
Children with HIV-seropositive caregivers were more likely to be tested if they were younger, a biological child, living with a widowed caregiver, and from an urban area Children with caregiver of a tribe other than the two large indigenous tribes, Chagga or Pare, were less likely to have been tested.
Biological children had more than five times the odds of being tested than non-biological children. Child testing was not predicted by whether the child was born in or the year of the caregiver’s HIV diagnosis. Testing in the HIV-seronegative group was associated with tribe only. In the community sample older children were more likely to be tested, and testing rates were higher if the caregiver had ever tested for HIV.
Discussion
Embedded in a study with adults with and without HIV infection, interviews included questions about HIV testing for their children. Overall, 31.6% of the children were reported to have been tested, including less than one-half of children with HIV-seropositive caregivers. Despite PEPFAR’s five-year goals for 65% early infant diagnosis and 80% of older children of HIV seropositive mothers by 2014 (OGAC, 2009 p. 6-9), it is apparent in these findings that child testing remains a major challenge in resource poor areas.
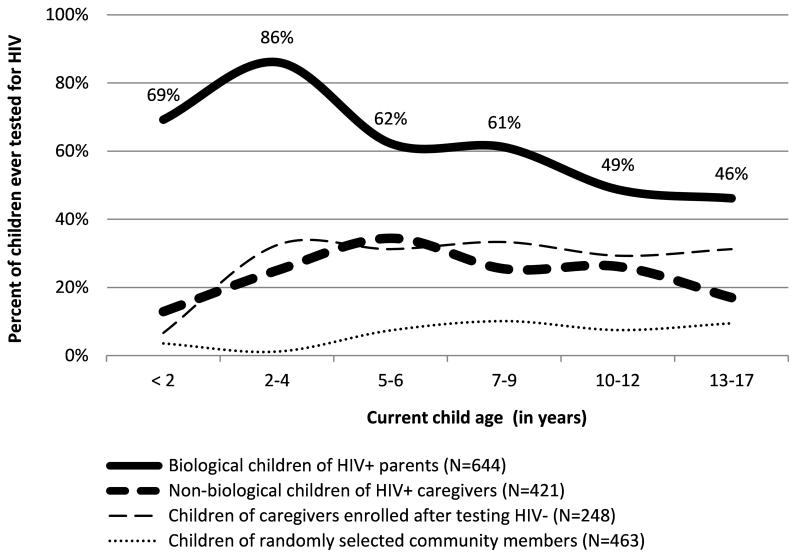
The UNAIDS Global Report 2010 indicated that the percent of pregnant women in Tanzania who received PMTCT was 70% (UNAIDS, 2010). In the Kilimanjaro region, PMTCT programs rolled out in 2004; and by the end of 2009, the use of DBS was widespread, including in rural clinics. Local data indicate that between October 2009 and September 2010 an estimated 83.5% of pregnant women were tested and received results. However, only 45.9% of all HIV exposed infants accessed EID services within 6 months of life (Schimana, Dow, Feng, Shayo, Bartlett, Mbita, Haule, Massambu, Charles, Buchanan, 2012). Accordingly, we performed a post hoc analysis for of child HIV testing rates by age. Of the 100 children who were less than 24 months at the time of the interview, 24 (24%) were reported to have been tested, 22 (38.6%) with HIV seropositive caregivers relative to 5% of children with HIV-seronegative caregivers (p<0.001.) Biological parents in the HIV-seropositive group reported that 18 of 26 children less than two were tested (69.2%). Nonetheless, higher and testing rates among younger children in the HIV-seropositive group could be indicative of a partial success of the scale-up of HIV testing in the region, although testing rates of 10% or less across ages in the community sample suggest that such effect may be limited to children with HIV positive parents. The relationship between child age and testing is detailed in Figure 1.
Figure 1. Rates of child HIV testing by current child age and caregiver HIV status (N=1,776).
Of note, tribal association other than the two large indigenous groups was associated with less child testing in both the HIV-seropositive and HIV-seronegative groups (see Table 2). Post hoc analysis found that over 30 tribes were represented in the “other tribe” group, each with very few numbers (range 1 to 22); and they are more likely to live in urban areas. It is speculated that these are, by and large, immigrated households that may live in more social isolation, with less knowledge about local services, and possibly more reluctance to risk possible discrimination related to HIV disclosure. The finding provides information for VCT education and outreach activities, especially in the urban centers.
Despite local assumptions about males in households being decision makers, there are no gender differences in ensuring child testing when controlling for other factors.
Barriers to the accessibility and acceptability of child testing are noted in prior research and input from local stakeholders in the Kilmanjaro region. Caregivers with infants born before the use of DBS in 2008/2009 were asked to return for infant testing at 18 months, and drop-out rates from PMTCT programs are known to be high (Manzi, Zacharish, Teck, et al., 2005). By the time a child is 18 months old, the caregiver may conclude that a child who appears healthy does not need to be evaluated further. Tanzania’s guidelines for infants in PMTCT programs include DBS testing at four to six weeks post-partum and six weeks after completion of breast feeding (or at 9 months if breastfeeding continues), still requiring a return visit to the PMTCT setting (Prevention of Mother-to-Child Transmission of HIV National Guidelines, 2011). Older children living with non-biological caregivers may be doing so because the biological parent has died; this is a high risk group often not tested.
Practices designed to improve child testing in LMIC include provider-initiated counseling and testing (PICT) and home based VCT. Numerous country and local plans have endorsed PITC (WHO, 2009) at routine health encounters; and studies have shown the practice to be acceptable by parents and guardians, especially when the mother is aware she is HIV-seropositive (Levin, Mathema, Stinson et al., 2012). Similarly, home based VCT is shown to be acceptable for adults and high risk children with home delivery of results critical for follow up (Sabapathy, van den Bergh, Fidler, et al., 2012; Tumwesigye, Wana, Kasasa et al., 2010).
Reluctance about child testing continues to be related not only to access but also to various issues with acceptability, such as cultural norms dictating that young infants should not be taken outside the house except for receipt of vaccines (not always co-located with EID), transportation, a child having to miss school, and the persisting stigma of HIV/AIDS and fear about discrimination (e.g. Kilewa, Massawe, Lyamuya et al., 2001; Parker & Aggleton, 2003; Castro and Farmer, 2005; Messer, Pence, Whetten et al., 2010). Women report being afraid to discuss their own diagnosis with their sexual partners for fear of violence (Rwemisisi, Wolff, Coutinho et al., 2008; Visser, 2012 p 6-9), and discussing HIV/AIDS with older children typically requires a discussion about sexual behavior (Poulsen, Miller, Lin et al., 2010).
This study has limitations, including data based on the report of caregivers. It is possible that the reporting caregiver did not know that the child had been tested, for example, if taken to a clinic by another parent or guardian. The interviews asked current child age in years, so the year “1” includes birth through 23 months; the birth to 12 month group may have shown a larger rate of testing given the timing of the scaling up of MCTC and DBS testing in the region. We did not ask about child age at testing, if tested.
Limitations notwithstanding, these data suggest that multiple intervention targets are needed to improve rates of child HIV testing and early treatment in LMIC. Scaling up resources for PMTCT, EID, and treatment availability does not necessarily translate to increased uptake of those resources. Specific barriers that continue despite improved access include difficult to address barriers such as stigma and discrimination and, especially for women, the threat of violence.
References
- Baggaley R, Hensen B, Ajose O, Grabbe KL, Wong VJ, Schilsky A, Lo YR, Granich R, Hargreaves J. From caution to urgency: the evolution of HIV testing and counseling in Africa. Bulletin of the World Health Organization. 2012;90(9):652–658B. doi: 10.2471/BLT.11.100818. doi:10.2471/BLT.11.100818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk DR, Falkovitz-Halpern MS, Hill DW, Albin C, Arrieta A, Bork JM, Cohan D, Nilson B, Petru A, Ruiz J, Weintrub P, Maldonado YA, for the California Pediatric HIV Study Group Temporal trends in early clinical manifestations of perinatal HIV infection in a population-based cohort. Journal of the American Medical Association. 2005;293:2221–2231. doi: 10.1001/jama.293.18.2221. [DOI] [PubMed] [Google Scholar]
- Castro A, Farmer P. Understanding and addressing AIDS-related stigma: from anthropological theory to clinical practice in Haiti. American Journal of Public Health. 2005;95(1):53–59. doi: 10.2105/AJPH.2003.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhagan MK, Sauchali S, Arpadi SM, Craib MH, Bah F, Stein Z, Davidson LL. Failure to test children of HIV-infected mothers in South Africa: implications for HIV testing strategies for preschool children. Tropical Medicine and International Health. 2011;16(12):1490–1494. doi: 10.1111/j.1365-3156.2011.02872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Lungu M, van Oosterhout JJ. HIV testing coverage of family members of adult antiretroviral therapy patients in Malawi. AIDS Care. 2010;22(11):1346–1349. doi: 10.1080/09540121003720986. [DOI] [PubMed] [Google Scholar]
- Dabis F, Ekpini ER. HIV-1/AIDS and maternal and child health in Africa. Lancet. 2002;359:2097–2104. doi: 10.1016/S0140-6736(02)08909-2. [DOI] [PubMed] [Google Scholar]
- De Cock, Fowler MG, Mercier E, de Vincenzi I, Saba J, Hoff E, Ainwick DJ, Rogers M, Shaffer N. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. Journal of the American Medical Association. 2000;283(9):1175–1182. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- Kellerman S, Essajee S. HIV testing for children in resource limited settings: What are we waiting for? PLos Medicine. 2010;7(7):e1000285. doi: 10.1371/journal.pmed.1000285. Doi:10.1371/journal.pmed.1000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilewo C, Massawe A, Lyamuya E, Semali I, Kalokola F, Urassa E, Giattas M, Temu F, Karlsson K, Mhalu F, Biberfeld G. HIV counseling and testing of pregnant women in Sub-Saharan Africa: Experiences from a study on prevention of mother to child HIV-1 transmission in Dar Es Salaam, Tanzania. Journal of Acquired Immune Deficiency Syndromes. 2001;28:458–462. doi: 10.1097/00042560-200112150-00009. [DOI] [PubMed] [Google Scholar]
- Levin M, Mathema H, Stinson K, Jennings K. Acceptability, feasibility, and impact of routine screening to detect undiagnosed HIV infection in 17-24 month old children in the western sub-district of Cape Town. South Africa Medical Journal. 2012;102:245–248. [PubMed] [Google Scholar]
- McCollum ED, Preidis GA, Colitko CL, Siwande LD, Mwansambo C, Kazembe PN, Hoffman I, Hosseinipour MC, Schutze GE, Kline MW. Routine inpatient Human Immunodeficiency Virus testing system increases access to pediatric Human Immunodeficiency Virus care in sub-Saharan Africa. Pediatric Infectious Diseases Journal. 2011;30(5):e75–e81. doi: 10.1097/INF.0b013e3182103f8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzi M, Zachariah R, Teck R, et al. High acceptability of voluntary counselling and HIV-testing but unacceptable loss to follow up in a prevention of mother-to-child transmission programme in rural Malawi: scaling up requires a different way of acting. Tropical Medicine and International Health. 2005;10(12):1242–1250. doi: 10.1111/j.1365-3156.2005.01526.x. [DOI] [PubMed] [Google Scholar]
- Messer L, Pence BW, Whetten K, Whetten R, Thielman N, O’Donnell K, Ostermann J. Prevalence and predictors of HIV-related stigma among institutional- and community-based caregivers of orphans and vulnerable children living in five less-wealthy countries. BMC Public Health. 2010;10z:504. doi: 10.1186/1471-2458-10-504. Retrieved 1 December 2012 at http://www.biomedcentral.com/1471-2458/10/504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health and Social Welfare. United Republic of Tanzania . Prevention of Mother-to-Child Transmission of HIV National Guidelines. 2nd edition Ministry of Health and Social Welfare; Dar es Saalam, TZ: 2011. [Google Scholar]
- Newell ML, Brahmbhatt H, Ghys PD. Child mortality and HIV infection in Africa: a Review. AIDS. 2004;18(Suppl.):27–34. doi: 10.1097/00002030-200406002-00004. [DOI] [PubMed] [Google Scholar]
- Newell ML, Coovadia H, Cortina-Borja M, Gaillard P, Dabis F, Ghent International AIDS Society (IAS) Working Group on HIV Infection in Women and Children Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. The Lancet. 2004;364(9441):1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- Parker R, Aggleton P. HIV and AIDS-related stigma and discrimination: a conceptual framework and implications for action. Social Science and Medicine. 2003;57:13–24. doi: 10.1016/s0277-9536(02)00304-0. [DOI] [PubMed] [Google Scholar]
- Poulsen MN, Miller KS, Lin C, Fasula A, Vandenhoudt H, Wyckoff SC, Ochura J, Obong’o CO, Forehand R. Factors associated with parent-child communication about HIV/AIDS in the United States and Kenya: a cross-cultural comparison. AIDS and Behavior. 2010;14(5):1083–94. doi: 10.1007/s10461-009-9612-4. doi: 10.1007/s10461-009-9612-4. [DOI] [PubMed] [Google Scholar]
- Rollins N, Mzolo S, Moodley T, Esterhuizen T, van Rooyen H. Universal HIV testing of infants at immunization clinics: an acceptable an feasible approach for early infant diagnosis in high HIV prevalence settings. AIDS. 2009;10(23):1851–1857. doi: 10.1097/QAD.0b013e32832d84fd. Doi: 10.1097/QAD.) 0b013e32832d84fd. [DOI] [PubMed] [Google Scholar]
- Rwemisisi J, Wolff B, Coutinho A, Grosskurth H, Whitworth J. ‘What if they ask how I got it?’ Dilemmas of disclosing parental HIV status and testing children for HIV in Uganda. Health Policy Plan. 2008;23(1):36–42. doi: 10.1093/heapol/czm040. [DOI] [PubMed] [Google Scholar]
- Sabapathy K, Van den Bergh R, Fidler S, Hayes R, Ford N. Uptake of Home-Based Voluntary HIV Testing in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. PLoS Med. 2012;9(12):e1001351. doi: 10.1371/journal.pmed.1001351. doi:10.1371/journal.pmed.1001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 12. StataCorp LP; College Station, TX: 2011. [Google Scholar]
- Turnwesigye E, Wana G, Kasasa S, Muganzi E, Nuwaha F. High uptake of home based, district-wide, HIV counseling and testing in Uganda. AIDS Patient Care and STDs. 2010;24(11):735–741. doi: 10.1089/apc.2010.0096. DOI:10.1089/apc.2010.0096. [DOI] [PubMed] [Google Scholar]
- UNAIDS . UNAIDS Report on the Global AIDS Epidemic 2012. Joint United National Program on HIV/AIDS; Geneva, Switzerland: 2012. [Google Scholar]
- UNICEF The State of the World’s Children 2009. 2009 http://www.unicef.org/sowc09/docs/SOWC09-FullReport-EN.pdf retrieved 1 December 2012.
- Violari A, Cotton MF, Gibb DM, Babiker Abdel, Steyn J, Madhi SA, Paed FC, Jean-Philippe P, McIntyre JA, for the CHER Study Team Early antiretroviral therapy and mortality among HIV-infected infants. New England Journal of Medicine. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker N, Schwartlander B, Bryce J. Meeting international goals in child survival and HIV/AIDS. Lancet. 2002;360:284–289. doi: 10.1016/S0140-6736(02)09550-8. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinsky M, Dewey JE. How to score and interpret single-item health status measures: a manual for users of the SF-8™ Health Survey. Quality Metric Incorporated; Lincoln, RI: 2001. [Google Scholar]
- World Health Organization . Towards Universal Access: Scaling up Priority HIV/AIDS Intervention in the Health Sector: Progress Report 2009. WHO; Geneva, Switzerland: 2009. [Google Scholar]