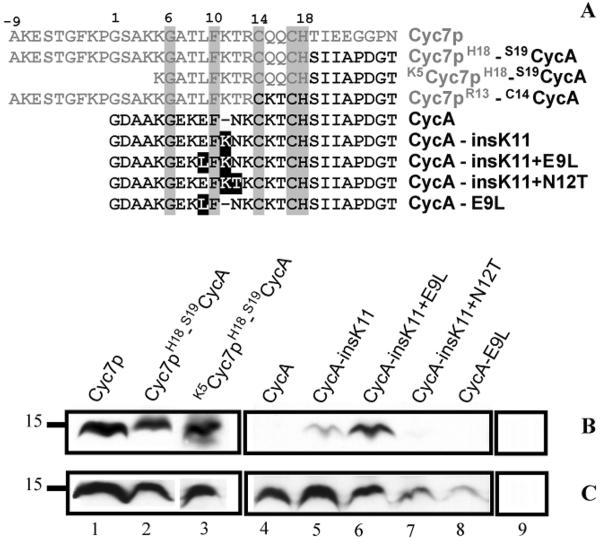
Figure 2.
R. capsulatus apocytochrome c2 (CycA) fusion and mutant proteins tested for CCHL-dependent holocytochrome c formation in E. coli. Amino-termini sequences of different chimeric proteins formed of S. cerevisiae iso-2-cytochrome c (Cyc7p) and R. capsulatus CycA, and CycA mutants used are shown in panel A. Amino acid residues originating from Cyc7p and CycA are depicted in grey and black, respectively. Of the cytochromes c used only eight amino acids immediately following the Cys-Xxx-Xxx-Cys-His heme binding site are depicted to show the junction sequence of the chimera. In the fusion proteins amino acid residues flanking each portion are indicated. Absolutely conserved amino acids are boxed in grey, and mutations in the CycA derivatives are boxed in black. Panels B and C show the heme staining and Strep-tag detection data, respectively, for various cytochromes c coproduced with CCHL (lanes 1–8) or for CCHL produced alone (lane 9) in E. coli EC06 (ΔCcm) using 75 μg of total proteins in each case. Molecular markers (kDa) are shown on the left.