Figure 4.
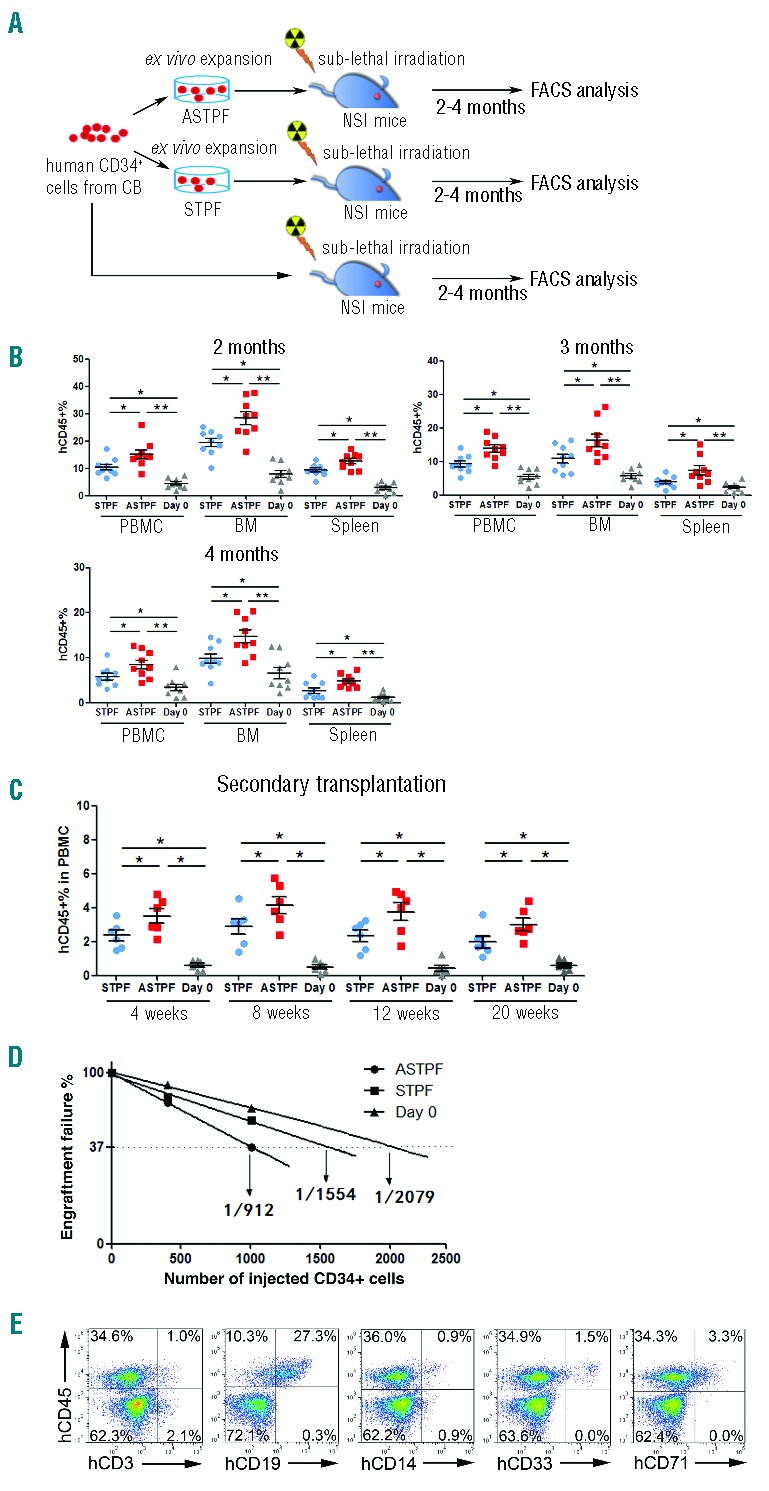
ANGPTL7 stimulates expansion of human hematopoietic stem and progenitor cells (HSPCs) ex vivo. (A) Experimental design for assessing the repopulation capacities of ex vivo expanded human HSPCs. Total of 1×104 fresh purified CD34+ human umbilical cord blood nucleated cells (hUCBNCs) and 1×104 purified CD34+ hUCBNCs cultured in ASTPF or STPF conditions for seven days were injected into three groups of sub-lethally irradiated NSI mice (9 mice per group). Two, three, and four months post transplantation, peripheral blood (PB), BM, and spleen (SP) samples from NSI mice from each group were subjected to FACS analysis. (B) Summary of percentages of human CD45+ cells in the PB, BM, and SP of NSI mice at two, three, or four months after injection with 1×104 purified CD34+ hUCBNCs or progeny of 1×104 purified CD34+ hUCBNCs that had been cultured in ASTPF or STPF conditions. Bars represent the mean percentages of human CD45+ cells in the PB, BM and SP of mice from each group (n=9). The experiments were repeated three times. *P≤0.05 for ASTPF group versus STPF group; **P≤0.01 for ASTPF group versus day 0 group; *P≤0.05 for STPF group versus day 0 group. (C) Bone marrow aspirate from one hind leg from a primary recipient was transplanted into 2 secondary recipients (n=6); *P≤0.05 for ASTPF group and STPF group versus day 0 group; ASTPF group versus STPF group. (D) Limiting-dilution analysis of the repopulating ability of cells (400 or 1000 cells) before culture (day 0) and after culture for seven days in STPF medium and ASTPF medium. Plots show percentage of recipient mice containing less than 1% human hematopoietic populations in recipient mouse bone marrow eight weeks after transplantation versus the number of input or input-equivalent cells injected (n=10 mice transplanted at each dose per condition; n=60 mice total). (E) Representative FACS analysis of percentages of multiple hematopoietic lineages in the BM of NSI mice from the ASTPF group two months post transplantation, as described in (B).
