Abstract
IMPORTANCE
Indwelling devices (eg, urinary catheters and feeding tubes) are often used in nursing homes (NHs). Inadequate care of residents with these devices contributes to high rates of multidrug-resistant organisms (MDROs) and device-related infections in NHs.
OBJECTIVE
To test whether a multimodal targeted infection program (TIP) reduces the prevalence of MDROs and incident device-related infections.
DESIGN, SETTING, AND PARTICIPANTS
Randomized clinical trial at 12 community-based NHs from May 2010 to April 2013. Participants were high-risk NH residents with urinary catheters, feeding tubes, or both.
INTERVENTIONS
Multimodal, including preemptive barrier precautions, active surveillance for MDROs and infections, and NH staff education.
MAIN OUTCOMES AND MEASURES
The primary outcome was the prevalence density rate of MDROs, defined as the total number of MDROs isolated per visit averaged over the duration of a resident's participation. Secondary outcomes included new MDRO acquisitions and new clinically defined device-associated infections. Data were analyzed using a mixed-effects multilevel Poisson regression model (primary outcome) and a Cox proportional hazards model (secondary outcome), adjusting for facility-level clustering and resident-level variables.
RESULTS
In total, 418 NH residents with indwelling devices were enrolled, with 34 174 device-days and 6557 anatomic sites sampled. Intervention NHs had a decrease in the overall MDRO prevalence density (rate ratio, 0.77; 95% CI, 0.62–0.94). The rate of new methicillin-resistant Staphylococcus aureus acquisitions was lower in the intervention group than in the control group (rate ratio, 0.78; 95% CI, 0.64–0.96). Hazard ratios for the first and all (including recurrent) clinically defined catheter-associated urinary tract infections were 0.54 (95% CI, 0.30–0.97) and 0.69 (95% CI, 0.49–0.99), respectively, in the intervention group and the control group. There were no reductions in new vancomycin-resistant enterococci or resistant gram-negative bacilli acquisitions or in new feeding tube–associated pneumonias or skin and soft-tissue infections.
CONCLUSIONS AND RELEVANCE
Our multimodal TIP intervention reduced the overall MDRO prevalence density, new methicillin-resistant S aureus acquisitions, and clinically defined catheter-associated urinary tract infection rates in high-risk NH residents with indwelling devices. Further studies are needed to evaluate the cost-effectiveness of this approach as well as its effects on the reduction of MDRO transmission to other residents, on the environment, and on referring hospitals.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01062841
Approximately 1.4 million Americans reside in 15 600 US nursing homes (NHs), and in 2010 there were 2.5 million short-stay admissions.1 More than 40% of all Medicare beneficiaries discharged from hospitals in 2011 required postacute care.2 Multidrug-resistant organisms (MDROs) are endemic in NHs, with prevalence rates exceeding 35% and surpassing those for hospitals.3–7 With increasing acuity of illness, the risk of acquiring new infections increases substantially, with approximately 2 million infections occurring in NHs each year.8
Indwelling devices, such as urinary catheters and feeding tubes, are frequently used in NHs.9–13 National data for the United States show that approximately 5% to 7% of all NH residents have an indwelling urinary catheter and that 5% to 7% have feeding tubes, while 12% to 15% of new admissions to NHs have an indwelling urinary catheter.1,9–12 Compared with NH residents without indwelling devices, residents with indwelling devices have a higher prevalence of MDRO colonization at multiple anatomic sites.3,14–16 The NH residents with indwelling devices share many characteristics with hospitalized populations, and improper care of residents with these devices presents opportunities for pathogen acquisition. The hands of health care workers (HCWs) in these NHs are frequently colonized with gram-negative bacilli (66%), Candida (41%), Staphylococcus aureus (20%), and vancomycin-resistant enterococci (VRE) (9%), increasing the risk of pathogen transmission when providing assistance with various activities of daily living.17
Despite the large number of NH residents who are colonized and subsequently infected, randomized trials evaluating interventions focused on the entire population or specific high-risk groups are infrequent.18,19 Studies conducted at single NHs have generally focused on 1 of the following 4 types of interventions: hand hygiene,17,20,21 gown use and contact precautions,22 decolonization regimens,23 or infection prevention education.24,25 Although these studies show a trend toward reduced MDRO colonization and infections, the reduction in MDROs has not been statistically significant in most cases. Therefore, we conducted a multicenter randomized clinical trial to test whether a multimodal practical evidence-based targeted infection program (TIP) would reduce the prevalence density of MDRO colonization and the incidence of new device-related infections in high-risk NH residents.
Methods
Design Overview, Setting, and Participants
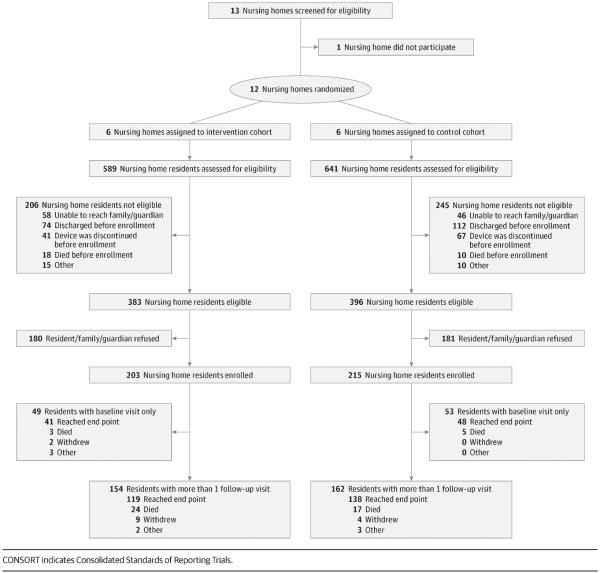
We approached 17 community-based NHs in southeast Michigan that had expressed a preliminary interest in participating in our research program (Figure). Thirteen agreed to have initial conversations with us, of which 12 agreed to participate and remained in our study for 3 years from May 2010 to April 2013. This study was approved by the institutional review board at the University of Michigan. The full study protocol can be found in the trial protocol in Supplement 1. All Medicare-certified and Medicaid-certified NHs have an infection control program.26 Similar to most US NHs, all study sites had an on-site infection preventionist, most of whom served in this role part-time and had additional responsibilities (eTable 1 in Supplement 2). All NHs had access to laboratory and radiology services.27 The TIP intervention included the following: (1) preemptive barrier precautions; (2) active surveillance for MDROs and infections, with data feedback; and (3) NH staff education on key infection prevention practices and hand hygiene promotion (Table 1). The control group NHs continued to practice according to their own infection prevention policies.
Figure.
CONSORT Diagram
Table 1.
Overview of Targeted Infection Prevention (TIP) Program Interventions
| Variable | Intervention Group (TIP) | Control Group (Usual Care) |
|---|---|---|
| Precautions | ||
|
| ||
| Enrolled residents (with urinary catheter and/or feeding tube) | Preemptive barrier precautions | Standard precautions |
| Glove and gown use for direct care | Transmission-based precautions as needed per NH policy | |
| Transmission-based precautions as needed per NH policy | ||
|
| ||
| Nonenrolled residents | Standard precautions | Standard precautions |
| Transmission-based precautions as needed per NH policy | Transmission-based precautions as needed per NH policy | |
|
| ||
| Surveillance | ||
|
| ||
| Multidrug-resistant organisms | Active surveillance | Passive surveillance |
| Cultures collected at baseline, day 15, and then monthly, with data reported back to the facilities every month | Cultures collected at baseline, day 15, and then monthly for outcome measurements only, with no reports given to the facilities | |
|
| ||
| Infections | Infections identified by study definitions and reported back to the facilities every month, along with reminders of key strategies to prevent infections | Infections identified by study definitions for outcome measurements only, with no reports given to the facilities |
|
| ||
| Education | ||
|
| ||
| NH staff | Hand hygiene promotion | Education provided as needed (eg, annual requirements, in response to state surveys or audits) |
| Posters, videos, Glo Germ gel (Glo Germ Company), pre-post cultures | ||
| Interactive infection prevention modules; ten sessions for health care workers | ||
| Infection surveillance pocket cards provided to nurse, nurse's aide, physician, and infection preventionist on resident enrollment | ||
| Half-day miniconference on infection surveillance; each NH infection preventionist was invited to participate | ||
Abbreviation: NH, nursing home.
Written informed consent to collect surveillance samples and resident-specific data was obtained from each resident or his or her durable power of attorney. Inclusion criteria were (1) any short-stay or long-stay resident with an indwelling urinary catheter, a feeding tube (nasogastric or percutaneous endoscopic gastrostomy tube), or both for more than 72 hours and (2) informed consent. Residents receiving end-of-life care were excluded. Study personnel (B.L., S.E.M., J.F., and E.K.) obtained data on participant characteristics, age, sex, functional status,28 comorbidity,29 and facility-level predictors, such as the quality ratings.30 The Centers for Medicare & Medicaid Services created the 5-star quality rating system to help consumers, their families, and caregivers compare NHs. This rating incorporates health inspections, staffing, and quality measures. While the field staff (B.L., S.E.M., J.F., and E.K.) were aware of the intervention assignment, one of us (K.S.) processing microbiology cultures was blinded.
Sample Size
Our sample included 12 NHs, with a mean of 137 beds each. The enrollment period for the study was 36 months. Based on preliminary data,9–11 we estimated that each NH would care for a mean of 22 residents with an indwelling device per year. Power calculations were based on anticipating a 30% reduction (rate ratio, 0.70) in MDRO prevalence as a result of our intervention. The desired statistical power was 80%, and the significance criterion was .05. Based on previous data,31 the expected MDRO prevalence density rates were 42 MDRO isolates, including new and repeat isolates from all anatomic sites, per 1000 device-days in the intervention group and 60 MDROs per 1000 device-days in the control group. Because this was a prospective study of NH residents, we also adjusted for intracluster correlation (using an intraclass correlation coefficient of 0.07).32–38 Therefore, 12 NHs were sufficient to reach 80% power to reject the null hypothesis of no difference in the overall MDRO prevalence density between the 2 groups. We anticipated conducting 1600 study visits over 3 years.
Randomization and Intervention
We chose to randomize NHs instead of participants using cluster randomization. Randomizing participants can lead to contamination by HCW experiences learned when caring for the intervention participants, thus underestimating the true effect of the intervention. Therefore, NHs served as the unit of allocation. The NHs were stratified by ownership (for profit vs not for profit) because prior studies39,40 have shown differences in quality of care by ownership status. Once stratified, NHs were assigned to intervention status using computer-generated randomization by our study statistician (A.G.). Six were randomized to the intervention group and 6 to the control group.
Precautions
Individuals in the intervention group with an indwelling device, irrespective of their MDRO colonization or infection status, were placed on preemptive barrier precautions for the duration of their participation. Specifically, barrier precaution signs were placed on the doors to their rooms, inside their closet, at the nurses' station, and on their medical records. The HCWs were encouraged to perform hand hygiene before and after providing any care to the participants and were encouraged to wear gowns and gloves when performing morning and evening care, device care, and during any care activity where splashing may be expected to occur. Hand hygiene products, disposable gowns, and personal protective equipment caddies were placed strategically at each intervention site. Residents were not isolated. They were allowed to socialize and obtain rehabilitation outside their room. For nonenrolled residents, intervention NHs followed their existing policies for those infected or colonized with MDROs.
Surveillance
Microbiological samples from the nares, oropharynx, enteral feeding tube insertion site, suprapubic catheter site, groin, peri-anal area, and wounds were obtained from all participants to assess MDRO colonization at baseline, day 15, and then monthly for up to 1 year, regardless of prior colonization status. Because there is no established active surveillance strategy in this diverse population with varying lengths of stay and case mix and because residents with devices have multisite colonization, we cultured multiple anatomic sites to assess our primary outcome.41–43 Standard microbiological methods were used to identify methicillin-resistant S aureus (MRSA), VRE, ceftazidime-resistant gram-negative bacilli, and ciprofloxacin-resistant gram-negative bacilli by trained research staff as previously described.31 Infections were identified using clinical definitions from participants' medical records, abstracted by trained study coordinators (B.L., S.E.M., and J.F.). The MDRO colonization and infection rates were reported monthly to the intervention NHs by the study coordinators. Graphs, charts, and tables of the data were designed to be easily understood by HCWs and emphasized infection and MDRO risks posed by indwelling devices.
Education
Once a resident was enrolled in the study, the nurse's aide, nurse, his or her physician of record, and the infection preventionist received informational pocket cards. The cards focused on infection recognition using NH-appropriate definitions, including the minimum criteria to initiate antibiotics for specific infections (eg, catheter-associated urinary tract infections [CAUTI])44 as well as criteria by McGeer et al45 for surveillance of infections (eAppendix in Supplement 2). Infection preventionists at the intervention NHs were also invited to a half-day conference on surveillance methods for infections (Conference Agenda in eAppendix in Supplement 2). In addition, a structured, interactive infection control education program for HCWs was designed, implemented, and evaluated. Ten educational modules, executed every 2 to 3 months over 3 years, were conducted by the research team (L.M., B.L., S.E.M., E.K., R.A.R., R.N.O., and S.F.B.) at the intervention NHs. The educational sessions were targeted to nurses and nurses' aides, although all NH staff were encouraged to attend. Each session was 30 minutes long, was offered multiple times to include workers on all shifts, and was accompanied by a pretest and posttest consisting of 5 to 10 questions. Session topics included an overview of infection prevention practices, hand hygiene, appropriate indications for device use and device care, and recognition of infections, with content following evidence-based guidelines. Each didactic session was presented using pretaped DVDs, followed by an interactive component that included game formats modeled after Jeopardy and Wheel of Fortune or device care skits on infection prevention themes. The HCWs at the control facilities were given a similar test on each session at the same time, but no education was provided.
Barrier precautions were promoted using posters, videos, and dance routines. The HCWs were educated on the indications, technique, and duration of effective hand hygiene as well as appropriate glove and gown use. To reduce the Hawthorne effect (also referred to as the observer effect), structured 30-minute observations to monitor HCW activities and their use of barrier precautions were conducted within participant rooms. The alternative of following an HCW in this setting would have changed his or her behavior.
Main Outcomes and Measures
The primary study outcome was the overall MDRO prevalence density rate, defined as each participant's total number of MDRO-positive anatomic sites across all MDROs per visit averaged over the duration of his or her participation. While incidence rates describing new acquisitions are appropriate for acute care hospitals, prevalence measures are better suited to describe the prevalence of multianatomic site MDRO colonization in a long-stay NH population from a public health perspective.42–44,46 This measure captures both transient and persistent MDRO colonization over the duration of participation (ie, a participant with persistent MDRO colonization would have a higher prevalence than someone with intermittent or no colonization). In addition, participants with a greater involvement of MDRO across anatomic sites would have a higher prevalence. We also evaluated the effect of this intervention on an individual's risk of new MDRO acquisition, defined as the number of residents with new acquisitions per 1000 device-days at risk as exploratory analyses. New acquisition was assessed at the resident level (eg, a resident culture negative for MRSA at baseline with a subsequent follow-up culture positive for MRSA at any anatomic site). The secondary outcome was the incidence of device-associated infections per 1000 device-days. We defined clinical infections objectively by the presence of both of the following: (1) a clinical note in the participant's medical record documenting an infection and (2) prescription of a systemic antibiotic for at least 3 days to treat that infection. Each participant was followed from enrollment up to 1 year or through his or her discharge, death, or device removal, whichever occurred first.
Statistical Analysis
A mixed-effects multilevel Poisson regression model was used to predict MDRO prevalence density as a function of the intervention (primary outcome), including facility as a random effect and offset by the number of anatomic sites sampled. To obtain the geometric mean, the prevalence measures were log transformed and then exponentiated back to the original units. Because we expected participants colonized at baseline to have a greater likelihood of subsequent colonization, we included participant-level baseline MDRO colonization as a fixed effect. The model was adjusted for participant-specific covariates (participant-level baseline colonization with the specific MDRO, age, sex, race, and length of stay before enrollment), and NH quality ratings.30 Using a Cox proportional hazards model, we conducted exploratory analyses to evaluate the effect of this intervention on an individual's risk of new MDRO acquisition, defined as the number of residents with new acquisitions per 1000 device-days at risk after adjusting for resident-level and facility-level covariates, as well as clustering by facility. Residents colonized with the specific MDRO at baseline were excluded from these analyses. For the secondary outcome of incident device-associated infections, a Cox proportional hazards model using the cluster option with robust standard errors was used to predict time to the first and recurrent infections, adjusting for participant-level and facility-level covariates. Residents with only a baseline visit and no follow-up visits were excluded from analysis.
Results
Study Group Characteristics
In total, 203 NH residents with indwelling devices were enrolled in the intervention group, with 17 490 device-days and 3283 active surveillance samples collected. In total, 215 NH residents were enrolled in the control group, with 16 684 device-days and 3274 samples collected. The exclusion of residents with baseline visits only and no follow-up resulted in an analytic population of 154 residents with 871 study visits in the intervention group (with 3116 samples collected) and 162 residents with 857 visits in the control group (with 3072 samples collected). There were no significant differences in baseline characteristics between these 2 groups (Table 2). The NH characteristics, assignments, and resident follow-up for both groups per Consolidated Standards of Reporting Trials guidelines47 are summarized in the Figure and in eTable 1 and eTable 2 in Supplement 2, respectively. The number of surveillance samples collected during the study and from specific anatomic sites did not differ between the 2 groups (eTable 3 and eTable 4 in Supplement 2).
Table 2.
Characteristics of Nursing Home Residents With Indwelling Devices
| Characteristic | All Residents | Residents With > 1 Follow-up Visit | ||||
|---|---|---|---|---|---|---|
| Intervention Group (n = 203) | Control Group (n = 215) | P Value | Intervention Group (n = 154) | Control Group (n = 162) | P Value | |
| No. of follow-up visits | 920 | 910 | NA | 871 | 857 | NA |
| Device-days, mean (SD) | 116.1(121.1) | 104.2(123.5) | .38 | 116.1(121.1) | 104.2(123.5) | .38 |
| Age, mean (SD),y | 74.4(12.4) | 72.5(13.2) | .14 | 74.0(12.4) | 72.2(13.5) | .22 |
| Male sex, No. (%) | 95 (46.8) | 123(57.2) | .03 | 76 (49.4) | 93 (57.4) | .15 |
| White race, No. (%) | 175 (86.2) | 187 (87.0) | .81 | 132(85.7) | 144 (88.9) | .39 |
| Comorbidity score, mean (SD) | 2.8(1.8) | 3.0(2.3) | .29 | 2.8(1.6) | 2.9(2.1) | .66 |
| Physical self-maintenance score, mean(SD) | 22.0(4.4) | 21.6(4.3) | .20 | 22.6(4.1) | 22.1(4.1) | .23 |
| Indwelling device use, No. (%) | .39 | .58 | ||||
| Urinary catheter | 120(59.1) | 112(52.1) | .18 | 84 (54.5) | 79 (48.8) | .30 |
| Feeding tube | 56(27.6) | 70(32.6) | .27 | 46 (29.9) | 54(33.3) | .50 |
| Both urinary catheter and feeding tube | 27(13.3) | 33(15.3) | .64 | 24(15.6) | 29(17.9) | .58 |
Abbreviation: NA, notapplicable.
MDRO Rates
The MDRO prevalence density rates based on cluster-level summaries are listed in eTable 5 in Supplement 2. Using data from the participants enrolled during the first 30 days, there was no statistically significant difference in the overall baseline MDRO colonization rates between the groups.
For the primary outcome, when adjusted for the cluster study design as well as for baseline colonization, age, sex, race, and NH quality rating, we found a 23% reduction in the prevalence density rate for all MDROs (rate ratio, 0.77; 95% CI, 0.62–0.94; P = .01) in the intervention group (Table 3). When analyzing data for individual MDROs, the intervention group had a 22% reduction in the MRSA prevalence density rate (rate ratio, 0.78; 95% CI, 0.64–0.96; P = .01). Participants with urinary catheters showed reduced colonization with MRSA and ceftazidime-resistant gram-negative bacilli, and participants with feeding tubes showed reduced colonization with MRSA. We also assessed the effectiveness of the TIP intervention in reducing MDRO colonization at different anatomic sites (eTable 6 in Supplement 2). When evaluating anatomic site-level differences for individual MDROs, the intervention group showed a lower mean MDRO prevalence (eTable 7 in Supplement 2)
Table 3.
Prevalence Rate of MDROs in Nursing Home Residents With Indwelling Devices Following a Targeted Infection Prevention Intervention
| Variable | No. of MDROs Isolated (%of Positive Swabs) | Value (95% Cl) | |||
|---|---|---|---|---|---|
| Intervention Group (n = 154) | Control Group (n = 162) | Cluster-Adjusted Rate Ratio, Algebraic Mean | Cluster- and Covariate-Adjusted Rate Ratio, Algebraic Mean | Cluster- and Covariate-Adjusted Rate Ratio, Geometric Mean | |
| All Indwelling Devices | |||||
| All MDROs | 1299 (26.6) | 1732(32.6) | 0.67(0.51–0.88)a | 0.65 (0.50–0.85)a | 0.77 (0.62–0.94)a |
| MRSA | 254(8.2) | 323(10.5) | 0.92(0.55–1.53) | 0.81(0.55–1.18) | 0.78 (0.64–0.96)a |
| VRE | 122 (3.9) | 162 (5.3) | 1.78(0.68–4.67) | 1.81 (0.72–4.55) | 1.20(0.82–1.75) |
| Ceftazidime-resistant GNB | 185 (5.4) | 295 (8.4) | 0.93(0.50–1.72) | 0.87(0.49–1.55) | 0.94(0.61–1.44) |
| Ciprofloxacin-resistant GNB | 738(19.5) | 952 (24.2) | 0.94(0.61–1.46) | 0.96(0.63–1.47) | 0.75 (0.58–0.97)a |
| Urinary Catheters | |||||
| All MDROs | 665 (27.0) | 784(32.8) | 0.64(0.50–0.83)a | 0.94(0.66–1.32) | 0.90(0.68–1.21) |
| MRSA | 141 (8.8) | 158(11.3) | 0.68(0.41–1.14) | 0.61 (0.40–0.93)a | 0.71 (0.58–0.86)a |
| VRE | 53(3.3) | 92 (6.6) | 1.54(0.60–3.96) | 1.45(0.61–3.43) | 0.85 (0.58–1.25) |
| Ceftazidime-resistant GNB | 89(5.2) | 112 (6.7) | 0.65 (0.41–1.02) | 0.58(0.39–0.87) | 0.57 (0.44–0.74)a |
| Ciprofloxacin-resistant GNB | 382 (19.8) | 422 (23.4) | 0.93(0.58–1.49) | 0.95(0.60–1.50) | 0.96(0.67–1.39) |
| Feeding Tubes | |||||
| All MDROs | 312(22.2) | 429 (26.9) | 0.79(0.45–1.38) | 0.77(0.45–1.31) | 0.91(0.55–1.50) |
| MRSA | 64 (6.4) | 86 (8.7) | 0.42 (0.22–0.80)a | 0.40(0.26–0.63)a | 0.56(0.38–0.82)a |
| VRE | 23(2.3) | 18(1.8) | 1.72(0.34–8.84) | 1.72(0.25–11.85) | 2.15(0.23–19.90) |
| Ceftazidime-resistant GNB | 29(2.4) | 71(6.1) | 1.79(0.34–9.44) | 2.15(0.79–5.88) | 1.24(0.27–5.63) |
| Ciprofloxacin-resistant GNB | 196(16.5) | 254(20.1) | 0.89(0.52–1.54) | 0.89(0.52–1.55) | 1.09(0.66–1.79) |
Abbreviations: GNB, gram-negative bacilli; MDROs, multi drug-resistant organisms; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
P < .05.
New acquisitions of MRSA and resistant gram-negative bacilli were common. For example, after excluding residents with baseline MRSA colonization, the rates of new acquisition were 6.2 and 7.9 per 1000 device-days at risk at intervention and control sites, respectively (Table 4). New VRE acquisition occurred less frequently. After excluding residents with baseline VRE colonization, the rates of new acquisition were 1.7 and 2.3 per 1000 device-days at risk at intervention and control sites, respectively. After accounting for facility-level and resident-level covariates as well as facility-level clustering, the hazard ratio for new MRSA acquisition was lower for the intervention group than for the control group (hazard ratio, 0.78; 95% CI, 0.65–0.95; P = .01). There were no differences in new VRE or resistant gram-negative bacilli acquisitions.
Table 4.
New Multidrug-Resistant Organism (MDRO) Acquisitiona
| Intervention Group | Control Group | Cluster- and Covariate-Adjusted Hazard Ratio (95% Cl) | P Value | ||||
|---|---|---|---|---|---|---|---|
| No. of Residents | Device-days at Riskb | Rate of New Acquisition per 1000 Device-days | No. of Residents | Device-days at Riskb | Rate of New Acquisition per 1000 Device-days | ||
| New MRSA Acquisition (n = 248 Residents at Risk)c | |||||||
| 54 | 8722 | 6.2 | 56 | 7115 | 7.9 | 0.78(0.65–0.95) | .01 |
| New VRE Acquisition (n = 258 Residents at Risk)c | |||||||
| 22 | 12 756 | 1.7 | 26 | 11070 | 2.3 | 0.85(0.45–1.60) | .61 |
| First New Ciprofloxacin-Resistant or Ceftazidime-Resistant GNB Acquisition (n = 211 Residents at Risk)c,d | |||||||
| 42 | 7524 | 5.6 | 35 | 5685 | 6.2 | 0.90(0.60–1.33) | .59 |
Abbreviations: GNB, gram-negative bacilli; MRSA,methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
New acquisition was assessed at the resident level (eg, a resident being negative for MRSA at baseline with a subsequent follow-up culture positive for MRSA at any anatomic site).
Once a participant was colonized with the MDRO, he or she did not contribute to at-risk days.
Excluded are participants with only 1 visit and those already colonized with the specific MDRO at baseline.
Included are first new ceftazidime-resistant GNB or ciprofloxacin-resistant GNB acquisition.
Incidence Rates of Device-Specific Infections
As summarized in Table 5, there were 56 new episodes of clinically defined CAUTIs in the intervention group over 9413 catheter-days, with an incidence rate of 5.9 per 1000 catheter-days. There were 75 new CAUTIs in the control group over 8118 catheter-days, with an incidence rate of 9.2 per 1000 catheter-days (cluster- and covariate-adjusted hazard ratio, 0.69; 95% CI, 0.49–0.99; P = .045). In total, 74 first new CAUTIs were observed, including 31 in the intervention group and 43 in the control group (crude incidence of CAUTIs, 5.2 and 10.0 per 1000 catheter-days, respectively). Based on the results of the Cox proportional hazards multivariable regression analyses, which focused on the first new clinically defined CAUTIs, the risk of having a CAUTI remained significantly lower in the intervention group than in the control group (hazard ratio, 0.54; 95% CI, 0.30–0.97; P = .04). There were much fewer feeding tube-associated pneumonias and skin and soft-tissue infections, with no differences between the intervention and control groups.
Table 5.
Clinically Defined Indwelling Device-Associated Infections in Nursing Home Residents
| Intervention Group | Control Group | Cluster-Adjusted Hazard Ratio (95% CI) | P Value | Cluster- and Covariate-Adjusted Hazard Ratio (95% Cl) | P Value | ||
|---|---|---|---|---|---|---|---|
| Infections | Device-days | Infections | Device-days | ||||
| First New CAUTI, All (n = 166 Residents) | |||||||
| 31 | 5982 | 43 | 4292 | 0.49 (0.27–0.90) | .02 | 0.54(0.30–0.97) | .04 |
| All New CAUTI, Includes Recurrent Infection (n = 166 Residents) | |||||||
| 56 | 9413 | 75 | 8118 | 0.62 (0.43–0.88) | .008 | 0.69 (0.49–0.99) | .045 |
| Feeding Tube-Associated Skin and Soft-Tissue Infection (n = 118 Residents) | |||||||
| 4 | 5635 | 3 | 5062 | 1.21 (0.43–3.43) | .72 | 1.09(0.22–5.45) | .92 |
| Feeding Tube-Associated Pneumonia (n = 118 Residents) | |||||||
| 10 | 5635 | 8 | 5062 | 1.09(0.36–3.26) | .88 | 1.83(0.53–6.31) | .34 |
Abbreviation: CAUTI, catheter-associated urinary tract infection.
Our implementation process was evaluated by assessing the use of NH-appropriate definitions to monitor infection rates, post-in-service HCW knowledge scores, and use of barrier precautions during high-risk activities, such as morning and evening care or when splashing was expected. These process measures are listed in eTable 8 in Supplement 2.
Discussion
We found that a multimodal evidence-based TIP intervention involving preemptive barrier precautions, active surveillance for infections and MDROs, hand hygiene promotion, and structured infection prevention education reduced colonization with MDROs and prevented clinically defined CAUTIs in our NH population with indwelling devices. We demonstrated the utility of using a comprehensive yet technically simple strategy to produce these outcomes instead of time-consuming and resource-intensive measures. Our strategies were aimed at enhancing the existing infection prevention program in NHs by making education interactive, multidimensional, and directly applicable to a high-risk population.
Several factors help explain the observed reduction in MDROs. First, preemptive barrier precautions likely led to reduced pathogen prevalence density and new MRSA acquisition. Instituting preemptive precautions has led to reduced MRSA, Clostridium difficile, and resistant gram-negative bacilli colonization in acute care, usually during MDRO outbreaks.48 Second, we complemented preemptive barrier precautions with interactive, engaging infection prevention education delivered through HCW in-service implementation. Informational slides were followed by creative interactive games that reinforced educational messages presented during the didactic sessions. Third, our intervention included interactive methods of enhancing hand hygiene, such as dance routines; demonstrations of hand washing technique (using Glo Germ gel; Glo Germ Company); and pre-post hand cultures, as well as environmental sampling to illustrate the transfer of organisms. These interactive strategies enhanced HCW knowledge and participation, facilitated conversations about infection prevention in NHs, and likely led to reduced transmission of pathogens when providing indwelling device care.
Our multimodal TIP intervention also led to a reduction in clinically defined CAUTIs, reflecting a reduction in antibiotic use as the intervention NHs adopted appropriate criteria for diagnosis of infections during the study period. Distribution of pocket cards that focused on infection recognition to all health care professionals involved in the resident care, including nurses' aides, nurses, and physicians, may have also affected rates by improving diagnosis of CAUTIs and reducing antibiotic use, leading to decreased prevalence density rates of MDROs. Educational sessions on appropriate urinary catheter use and care (including emphasis on hand hygiene) may have reduced CAUTI rates by limiting contamination of catheter sites. In the Netherlands, Cools and van der Meer49 established an overall infection control program in one NH, with emphasis on hand hygiene, restriction of long-term indwelling urethral catheters, treatment of only symptomatic infections, and weekly all-physician meetings to discuss residents with infections. That program reduced clinically defined urinary tract infections from 256 in 515 residents in year 1 to 66 in 527 residents in year 6. In a more recent study, Evans et al25 introduced an MRSA bundle of universal nasal surveillance on admission, transfer, or discharge, as well as contact precautions for MRSA infection or colonization, hand hygiene promotion, and institutional culture change. Their program resulted in a trend toward reducing MRSA CAUTIs in Veterans Affairs spinal cord injury units. Ours is one of the first studies involving multiple community-based NHs in the United States to show the effectiveness of a multimodal TIP intervention in reducing antibiotic-treated CAUTIs.
The key strengths of this study are the evaluation of a multimodal TIP intervention in NHs and the goal to reduce MDRO colonization and infections in a high-risk population with indwelling devices. The TIP components were designed to upgrade and integrate individual practices into routine NH care, with emphasis on appropriate use and harms related to the use of indwelling devices. We examined a high-risk population in which there is a lack of rigorous randomized clinical trials to guide clinical practice and policy.17 Ours is also one of the initial studies involving a community-based NH consortium showing that horizontal interventions to enhance routine infection prevention practices reduce MDRO colonization and antibiotic use related to CAUTIs in a high-risk population.
The study also has limitations. First, our study was conducted in NH facilities in southeast Michigan. While our study facilities resemble other US NHs with respect to their ownership status, the mean number of beds, availability of laboratory and radiology services, and the presence of a person responsible for infection prevention, our results may not be generalizable to other types of long-term care facilities, such as assisted-living facilities and hospice centers. Second, we did not assess the effect of the TIP intervention on other potentially at-risk residents at these NHs (eg, residents with wounds). With the increasing complexity of resident populations being served at these NHs, future studies should target other at-risk groups. Our goal was to evaluate the effectiveness of our TIP intervention in reducing MDRO transmission from HCWs to a population at high risk of MDRO acquisition due to the presence of indwelling devices. Whether these interventions lead to reduced transmission from these high-risk residents to the rest of the population also needs to be evaluated in the future. Third, we defined CAUTIs using clinical definitions. A recent systematic review demonstrates that there are many different definitions for CAUTIs used in the long-term care setting.50 Ninety percent of all controlled interventional studies reported either symptomatic or physician-diagnosed urinary tract infections (n = 13) or CAUTI definition not specified (n = 5) as the outcome of interest. We chose to use clinically defined CAUTIs because this measure represents actual antibiotic-treated infections, and antibiotic use remains a significant risk factor for MDRO colonization. Fourth, we did not monitor hand hygiene that could have occurred outside a resident's room; therefore, our estimates of in-room hand hygiene compliance may be conservative. To minimize the Hawthorne effect, research personnel conducted in-room observations instead of following HCWs as they conducted their duties.51 Fifth, we did not assess the cost-effectiveness of the interventions because it was beyond the scope of this study.
Conclusions
Limitations notwithstanding, we found that a comprehensive multimodal TIP intervention in NHs focused on reducing MDRO colonization and CAUTIs is feasible and effective. We designed the TIP intervention by engaging community-based NHs with little experience in conducting prospective research and with few infection prevention resources to demonstrate strategies that could be adopted in this traditionally resource-poor setting.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by RO1AG032298 from the National Institute on Aging (Drs Mody, Krein, Saint, Galecki, Kauffman, and Bradley), by K23AG028943 from the National Institutes of Health (Dr Mody), by RO1AG041780 from the National Institute on Aging (Drs Mody, Min, Bradley), and by the Claude D. Pepper Older American Independence Centers funding from the National Institute on Aging (Dr Mody).
Role of the Funder/Sponsor: The sponsors of the study had no role in the design, data gathering, analysis, interpretation, writing of the report, or decision to submit the article for publication.
Footnotes
Author Contributions: Dr Mody had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Mody, Krein, Saint, Galecki, Kauffman, Bradley.
Acquisition, analysis, or interpretation of data: Mody, Min, Lansing, McNamara, Symons, Fisch, Koo, Galecki, Kabeto, Fitzgerald.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Mody, Min, Galecki, Kabeto.
Obtained funding: Mody, Krein, Saint, Galecki, Kauffman, Bradley.
Administrative, technical, or material support: Mody, Lansing, McNamara, Fisch, Rye, Fitzgerald, Olmsted.
Conflict of Interest Disclosures: None reported.
Additional Contributions: With approval of the National Institute on Aging, GOJO Industries, Inc donated personal-use hand sanitizer bottles to the intervention sites.
Supplemental content at jamainternalmedicine.com
REFERENCES
- 1.Centers for Medicare & Medicaid Services [Accessed December 3, 2014];Nursing Home Data Compendium. 2013 http: //www.cms.gov/Medicare/Provider-Enrollment -and-Certification/CertificationandComplianc/downloads/nursinghomedatacompendium_508.pdf.
- 2.Medicare Payment Advisory Commission [Accessed November 26, 2014];Report to the Congress: Medicare and the health care delivery system. Statement of Mark E. Miller, Ph.D. 2014 Jun 18; http://www.medpac.gov/documents/congressional-testimony/testimony-report-to-the -congress-medicare-and-the-health-care-delivery -system-(ways-and-means-june-18-2014).pdf?sfvrsn=0.
- 3.Mody L, Kauffman CA, Donabedian S, Zervos M, Bradley SF. Epidemiology of Staphylococcus aureus colonization in nursing home residents. Clin Infect Dis. 2008;46(9):1368–1373. doi: 10.1086/586751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds C, Quan V, Kim D, et al. Methicillin-resistant Staphylococcus aureus (MRSA) carriage in 10 nursing homes in Orange County, California. Infect Control Hosp Epidemiol. 2011;32(1):91–93. doi: 10.1086/657637. [DOI] [PubMed] [Google Scholar]
- 5.Morgan DJ, Day HR, Furuno JP, et al. Improving efficiency in active surveillance for methicillin-resistant Staphylococcus aureus or vancomycin-resistant Enterococcus at hospital admission. Infect Control Hosp Epidemiol. 2010;31(12):1230–1235. doi: 10.1086/657335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419–1430. doi: 10.1056/NEJMoa1007474. [DOI] [PubMed] [Google Scholar]
- 7.Bonomo RA. Multiple antibiotic-resistant bacteria in long-term-care facilities: an emerging problem in the practice of infectious diseases. Clin Infect Dis. 2000;31(6):1414–1422. doi: 10.1086/317489. [DOI] [PubMed] [Google Scholar]
- 8.Strausbaugh LJ, Joseph CL. The burden of infection in long-term care. Infect Control Hosp Epidemiol. 2000;21(10)::674–679. doi: 10.1086/501712. [DOI] [PubMed] [Google Scholar]
- 9.Tsan L, Davis C, Langberg R, et al. Prevalence of nursing home-associated infections in the Department of Veterans Affairs nursing home care units. Am J Infect Control. 2008;36(3):173–179. doi: 10.1016/j.ajic.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Tsan L, Langberg R, Davis C, et al. Nursing home-associated infections in Department of Veterans Affairs community living centers. Am J Infect Control. 2010;38(6):461–466. doi: 10.1016/j.ajic.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Rogers MA, Mody L, Kaufman SR, Fries BE, McMahon LF, Jr, Saint S. Use of urinary collection devices in skilled nursing facilities in five states. J Am Geriatr Soc. 2008;56(5):854–861. doi: 10.1111/j.1532-5415.2008.01675.x. [DOI] [PubMed] [Google Scholar]
- 12.Teno JM, Feng Z, Mitchell SL, Kuo S, Intrator O, Mor V. Do financial incentives of introducing case mix reimbursement increase feeding tube use in nursing home residents? J Am Geriatr Soc. 2008;56(5):887–890. doi: 10.1111/j.1532-5415.2008.01647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell SL, Teno JM, Roy J, Kabumoto G, Mor V. Clinical and organizational factors associated with feeding tube use among nursing home residents with advanced cognitive impairment. JAMA. 2003;290(1):73–80. doi: 10.1001/jama.290.1.73. [DOI] [PubMed] [Google Scholar]
- 14.Mody L, Maheshwari S, Galecki A, Kauffman CA, Bradley SF. Indwelling device use and antibiotic resistance in nursing homes: identifying a high-risk group. J Am Geriatr Soc. 2007;55(12):1921–1926. doi: 10.1111/j.1532-5415.2007.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, Enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med. 2002;136(11):834–844. doi: 10.7326/0003-4819-136-11-200206040-00013. [DOI] [PubMed] [Google Scholar]
- 16.Rogers MA, Mody L, Chenoweth C, Kaufman SR, Saint S. Incidence of antibiotic-resistant infection in long-term residents of skilled nursing facilities. Am J Infect Control. 2008;36(7):472–475. doi: 10.1016/j.ajic.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mody L, McNeil SA, Sun R, Bradley SE, Kauffman CA. Introduction of a waterless alcohol-based hand rub in a long-term-care facility. Infect Control Hosp Epidemiol. 2003;24(3):165–171. doi: 10.1086/502185. [DOI] [PubMed] [Google Scholar]
- 18.Smith PW, Bennett G, Bradley SF, et al. SHEA/APIC guideline: infection prevention and control in long-term care facilities. Infect Control Hosp Epidemiol. 2008;29:785–814. doi: 10.1086/592416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchida M, Pogorzelska-Maziarz M, Smith PW, Larson E. Infection prevention in long-term care: a systematic review of randomized and nonrandomized trials. J Am Geriatr Soc. 2013;61(4):602–614. doi: 10.1111/jgs.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chami K, Gavazzi G, Bar-Hen A, et al. A short-term, multicomponent infection control program in nursing homes: a cluster randomized controlled trial. J Am Med Dir Assoc. 2012;13(6):569.e9–569.e17. doi: 10.1016/j.jamda.2012.04.008. doi:10.1016/j.jamda.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Schweon SJ, Kirk J. A realistic approach towards hand hygiene for long-term care residents and health care personnel. Am J Infect Control. 2011;39(4):336–338. doi: 10.1016/j.ajic.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Trick WE, Weinstein RA, DeMarais PL, et al. Comparison of routine glove use and contact-isolation precautions to prevent transmission of multidrug-resistant bacteria in a long-term care facility. J Am Geriatr Soc. 2004;52(12):2003–2009. doi: 10.1111/j.1532-5415.2004.52555.x. [DOI] [PubMed] [Google Scholar]
- 23.Wendt C, Schinke S, Württemberger M, Oberdorfer K, Bock-Hensley O, von Baum H. Value of whole-body washing with chlorhexidine for the eradication of methicillin-resistant Staphylococcus aureus: a randomized, placebo-controlled, double-blind clinical trial. Infect Control Hosp Epidemiol. 2007;28(9):1036–1043. doi: 10.1086/519929. [DOI] [PubMed] [Google Scholar]
- 24.Baldwin NS, Gilpin DF, Tunney MM, et al. Cluster randomised controlled trial of an infection control education and training intervention programme focusing on methicillin-resistant Staphylococcus aureus in nursing homes for older people. J Hosp Infect. 2010;76(1):36–41. doi: 10.1016/j.jhin.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Evans CT, Hill JN, Guihan M, et al. Implementing a patient education intervention about methicillin-resistant Staphylococcus aureus prevention and effect on knowledge and behavior in veterans with spinal cord injuries and disorders: a pilot randomized controlled trial. J Spinal Cord Med. 2014;37(2):152–161. doi: 10.1179/2045772313Y.0000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Medicare & Medicaid Services [Accessed December 3, 2014];CMS Manual System. http://www.cms.gov/Regulations -and-Guidance/Guidance/Transmittals/downloads /r55soma.pdf.
- 27.Young Y, Barhydt NR, Broderick S, Colello AD, Hannan EL. Factors associated with potentially preventable hospitalization in nursing home residents in New York State: a survey of directors of nursing. J Am Geriatr Soc. 2010;58(5):901–907. doi: 10.1111/j.1532-5415.2010.02804.x. [DOI] [PubMed] [Google Scholar]
- 28.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Medicare & Medicaid Services [Accessed May 29, 2013];Nursing Home Compare. http://www.medicare.gov/nursinghomecompare/search.html.
- 31.Wang L, Lansing B, Symons K, et al. Infection rate and colonization with antibiotic-resistant organisms in skilled nursing facility residents with indwelling devices. Eur J Clin Microbiol Infect Dis. 2012;31(8):1797–1804. doi: 10.1007/s10096-011-1504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donner A, Klar NS. Design and Analysis of Cluster Randomisation Trials in Health Research. Arnold; London, England: 2000. pp. 64–69. [Google Scholar]
- 33.Medical Research Council . Cluster Randomized Trials: Methodological and Ethical Considerations. Medical Research Council; London, England: 2002. (MRC Clinical Trials Series). [Google Scholar]
- 34.Ray WA, Taylor JA, Meador KG, et al. A randomized trial of a consultation service to reduce falls in nursing homes. JAMA. 1997;278(7):557–562. [PubMed] [Google Scholar]
- 35.Loeb MB. Application of the development stages of a cluster randomized trial to a framework for valuating complex health interventions. BMC Health Serv Res. 2002;2(1):13. doi: 10.1186/1472-6963-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loeb M, Carusone SC, Goeree R, et al. Effect of a clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial. JAMA. 2006;295(21):2503–2510. doi: 10.1001/jama.295.21.2503. [DOI] [PubMed] [Google Scholar]
- 37.Kerry SM, Bland JM. Sample size in cluster randomisation. BMJ. 1998;316(7130):549. doi: 10.1136/bmj.316.7130.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol. 1999;28(2):319–326. doi: 10.1093/ije/28.2.319. [DOI] [PubMed] [Google Scholar]
- 39.Hillmer MP, Wodchis WP, Gill SS, Anderson GM, Rochon PA. Nursing home profit status and quality of care: is there any evidence of an association? Med Care Res Rev. 2005;62(2):139–166. doi: 10.1177/1077558704273769. [DOI] [PubMed] [Google Scholar]
- 40.Harrington C, Woolhandler S, Mullan J, Carrillo H, Himmelstein DU. Does investor ownership of nursing homes compromise the quality of care? Am J Public Health. 2001;91(9):1452–1455. doi: 10.2105/ajph.91.9.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisch J, Lansing B, Wang L, et al. New acquisition of antibiotic-resistant organisms in skilled nursing facilities. J Clin Microbiol. 2012;50(5):1698–1703. doi: 10.1128/JCM.06469-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwaber MJ, De-Medina T, Carmeli Y. Epidemiological interpretation of antibiotic resistance studies: what are we missing? Nat Rev Microbiol. 2004;2(12):979–983. doi: 10.1038/nrmicro1047. [DOI] [PubMed] [Google Scholar]
- 43.Dulon M, Haamann F, Peters C, Schablon A, Nienhaus A. MRSA prevalence in European healthcare settings: a review. BMC Infect Dis. 2011;11:138. doi: 10.1186/1471-2334-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loeb M, Bentley DW, Bradley S, et al. Development of minimum criteria for the initiation of antibiotics in residents of long-term-care facilities: results of a consensus conference. Infect Control Hosp Epidemiol. 2001;22(2):120–124. doi: 10.1086/501875. [DOI] [PubMed] [Google Scholar]
- 45.McGeer A, Campbell B, Emori TG, et al. Definitions of infection for surveillance in long-term care facilities. Am J Infect Control. 1991;19(1):1–7. doi: 10.1016/0196-6553(91)90154-5. [DOI] [PubMed] [Google Scholar]
- 46.Cohen AL, Calfee D, Fridkin SK, et al. Society for Healthcare Epidemiology of America and the Healthcare Infection Control Practices Advisory Committee Recommendations for metrics for multidrug-resistant organisms in healthcare settings: SHEA/HICPAC position paper. Infect Control Hosp Epidemiol. 2008;29(10):901–913. doi: 10.1086/591741. [DOI] [PubMed] [Google Scholar]
- 47.Campbell MK, Piaggio G, Elbourne DR, Altman DG, CONSORT Group CONSORT 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661. doi: 10.1136/bmj.e5661. doi:10.1136/bmj.e5661. [DOI] [PubMed] [Google Scholar]
- 48.Safdar N, Marx J, Meyer NA, Maki DG. Effectiveness of preemptive barrier precautions in controlling nosocomial colonization and infection by methicillin-resistant Staphylococcus aureus in a burn unit. Am J Infect Control. 2006;34(8):476–483. doi: 10.1016/j.ajic.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Cools HJ, van der Meer JW. Infection control in a skilled nursing facility: a 6-year survey. J Hosp Infect. 1988;12(2):117–124. doi: 10.1016/0195-6701(88)90134-x. [DOI] [PubMed] [Google Scholar]
- 50.Meddings J, Mody L, Gaies E, Hickner A, Saint S. [Accessed January 24, 2015];What interventions are effective in preventing catheter-associated urinary tract infections in long-term care settings? PROSPERO. 2013 :CRD42013005787. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID= CRD42013005787.
- 51.Kohli E, Ptak J, Smith R, Taylor E, Talbot EA, Kirkland KB. Variability in the Hawthorne effect with regard to hand hygiene performance in high-and low-performing inpatient care units. Infect Control Hosp Epidemiol. 2009;30(3):222–225. doi: 10.1086/595692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.