Abstract
The orexin/hypocretin system is involved in multiple cocaine addiction processes that involve drug-associated environmental cues, including cue-induced reinstatement of extinguished cocaine seeking and expression of conditioned place preference. However, the orexin system does not play a role in several behaviors that are less cue-dependent, such as cocaine-primed reinstatement of extinguished cocaine seeking and low-effort cocaine self-administration. We hypothesized that cocaine-associated cues, but not cocaine alone, engage signaling at orexin-1 receptors (OX1R), and this cue-engaged OX1R signaling increases motivation for cocaine. Motivation for cocaine was measured in Sprague-Dawley rats with behavioral-economic demand curve analysis after pretreatment with the OX1R antagonist SB-334867 (SB) or vehicle with and without light+tone cues. Demand for cocaine was higher when cocaine-associated cues were present, and SB only reduced cocaine demand in the presence of these cues. We then asked if cocaine demand is linked to cued-reinstatement of cocaine seeking, as both procedures are partially driven by cocaine-associated cues in an orexin-dependent manner. SB blocked cue-induced reinstatement behavior, and baseline demand predicted SB efficacy with the largest effect in high demand animals, i.e., animals with the greatest cue-dependent behavior. We conclude that OX1R signaling increases the reinforcing efficacy of cocaine-associated cues but not for cocaine alone. This supports our view that orexin plays a prominent role in the ability of conditioned cues to activate motivational responses.
Keywords: Relapse, Self-administration, Behavioral economics, Demand curve, Elasticity
Introduction
Orexin-1 receptor (OX1R) signaling has been implicated in cocaine abuse (Mahler et al., 2012). Specifically, OX1Rs are involved in behaviors dependent on cocaine-associated cues, such as expression of conditioned place preference (Harris et al., 2005; Sartor & Aston-Jones, 2012) and relapse to cocaine seeking in response to cocaine-associated cues (Smith et al., 2009; 2010; James et al., 2011; Mahler et al., 2013). In contrast, OX1R signaling is not involved in behaviors that are less cue-dependent, such as low-effort self-administration or cocaine-primed reinstatement of cocaine seeking (Deroche-Gamonet et al., 2002; Smith et al., 2009; Mahler et al., 2013). The role of OX1Rs in only specific cocaine-seeking behaviors may be explained in part by OX1R signaling in ventral tegmental area (VTA). OX1R signaling in VTA potentiates glutamatergic responses of dopamine neurons (Borgland et al., 2009; Moorman & Aston-Jones, 2010), and this potentiation is necessary for cued-induced, but not cocaine-primed, reinstatement of cocaine seeking (Mahler et al., 2013). Similarly, OX1R signaling in VTA is necessary for high-effort responding for cocaine (España et al., 2010; 2011); however, considering that OX1R signaling is not necessary for prime-induced relapse of cocaine seeking, it is unclear how OX1R signaling augments motivation for cocaine.
We hypothesized that OX1R signaling is engaged by cocaine-associated cues and that this augments high-effort responding for cocaine; i.e., the apparent role of OX1R signaling in motivation for cocaine is accounted for by cue-dependent orexin release that augments motivation for cocaine. To test this we assessed the role of OX1R signaling in the reinforcing efficacy of cocaine and cocaine-associated cues with behavioral-economic demand curve analyses. In this approach drug demand (consumption) is measured as a function of price, determined here as the number of lever responses required to receive a mg of cocaine. The efficacy of the reinforcer delivered is specified by the effort an animal will expend to maintain drug consumption established at low prices.
We predicted that cocaine-associated cues would increase drug consumption at high, but not low, prices and that this would be OX1R-dependent. Drug-associated cues can take on motivational properties that drive behavior independently of the presence of the unconditioned drug reinforcer (Robinson et al., 2014); thus, both cocaine and response-contingent cocaine-associated cues may contribute to the total reinforcing efficacy of self-administered cocaine. As the price of cocaine increases throughout the session, the proportional contribution of cues to the total reinforcing efficacy should also increase. We hypothesized that if OX1R signaling contributes to the reinforcing efficacy of drug-associated cues, then an OX1R antagonist would have a larger relative effect on cocaine consumption at higher prices, and this effect would only be present when cocaine-associated cues were present. Also, if both demand and cued-reinstatement of drug seeking are driven in part by drug cues, then we expected to find an association between demand and cue-induced reinstatement of drug seeking (Galuska et al., 2011; Bentzley et al., 2014). Furthermore, we predicted that the reduction in cue-induced reinstatement with OX1R blockade would be more pronounced in animals with the highest demand for cocaine, as these animals’ behavior is likely more cue-dependent.
Materials and Methods
Animals
Male Sprague-Dawley rats (n = 28) with an initial weight of approximately 250–275g (Charles River, Raleigh, NC, USA) were single-housed under a reversed 12-h light/dark cycle (lights on 6 p.m.) with ad libitum access to food and water. Animals were housed in a temperature and humidity-controlled animal facility at Medical University of South Carolina (MUSC; AAALAC-accredited). All experiments were approved by the Institutional Animal Care and Use Committee at MUSC and conducted according to specifications of the National Institutes of Health as outlined in the Guide for the Care and Use of Laboratory Animals.
Intravenous catheter surgery
Animals were acclimated to the housing facilities and implanted with intravenous jugular catheters as previously described (Smith et al., 2009). Animals were anesthetized prior to surgery using a ketamine/xylazine mixture (56.6/8.7 mg/kg i.p.). After obtaining a deep plane of anesthesia (lack of corneal reflex), the free end of the catheter was inserted into the right external jugular vein. The tubing was run subcutaneously and exited through the skin via a biopsy hole placed 2 cm caudal to the mid-scapular region. Meloxicam was administered as a post-surgical analgesic (1 mg/kg, s.c.). Beginning 3 days after surgery, catheters were flushed daily with 0.1 ml of heparin (100 mg/mL) and 0.1 ml of cefazolin (100 U/mL). Animals were allowed to recover for at least 1 week before cocaine self-administration training.
Drugs
Cocaine HCl powder was provided by the National Institute on Drug Abuse (NIDA; Research Triangle Park, NC, USA) and was dissolved in 0.9% sterile saline. As previously reported (Smith et al., 2009), SB-334867 (SB) [1-(2-methylbenzoxazol-6-yl)-3-[1,5]naphthyridin-4-yl urea hydrochloride; provided by NIDA] was suspended in 2% dimethylsulfoxide and 10% 2-hydroxypropyl-b-cyclodextrin (Sigma) in sterile water; 0 or 30 mg/kg was given in a volume of 4 ml/kg (i.p.) 30 min prior to testing. SB has 50-fold selectivity for OX1R over OX2R and 100-fold selectivity over approximately 50 other molecular targets (Porter et al., 2001; Smart et al., 2001).
Behavioral economics theory
We tested the role of OX1R signaling in motivation for cocaine using behavioral-economic demand curve analysis. Briefly, this approach yields two distinct measures of cocaine-self administration: Q0 is a measure of drug consumption at null effort and α (demand elasticity) is an inverse measure of motivation. Notably, α is a unique measure of motivation in that it is independent of reinforcer dose and animal tolerance to drug effects, distinguishing it from other measures of motivation such as progressive ratio breakpoint (Hursh & Winger, 1995; Hursh & Silberberg, 2008; Bentzley et al., 2013; 2014). These measures of cocaine demand are determined here using a within-session threshold procedure (Oleson et al., 2011) coupled to a method of demand curve analysis that we recently developed (Bentzley et al., 2013). In this task demand for cocaine (consumption) is measured across increasing cocaine prices (lever responses/mg cocaine) by successively decreasing cocaine infusion doses in 10-min bins (Oleson et al., 2011). An exponential demand equation (Hursh & Silberberg, 2008) is then fit to each animal’s results (Bentzley et al., 2013) to determine baseline economic demand for cocaine. Demand parameters Q0 and α are extracted from the resulting demand curves and describe drug consumption at null cost (Q0; free consumption) and rate of consumption decline with increasing price (α; demand elasticity) respectively (Hursh & Silberberg, 2008). Importantly, α (also referred to as “essential value”) scales inversely with motivation for drug, as animals with high demand elasticity (high α) demonstrate rapid reductions in drug consumption with increases in drug price. Furthermore, α is a measure of demand elasticity that is normalized with respect to free drug consumption (Q0). The normalized nature of α enables motivation for drug to be tracked independently of changes in drug tolerance or sensitivity, because α represents an intrinsic property of the drug’s motivational efficacy, independent of the animal’s preferred drug consumption at null cost, Q0 (Hursh & Silberberg, 2008; Bentzley et al., 2014).
Self-administration and threshold procedure
Self-administration sessions were carried out in operant conditioning chambers housed in sound-attenuating cubicles and controlled by a MED-PC IV program (Med-Associates, St Albans, VT, USA). Before beginning the threshold procedure, rats learned to lever-press for 0.19 mg infusions of intravenous cocaine on a fixed-ratio-1 (FR-1) schedule in 1-hr daily sessions. Infusions were delivered over 3.6 sec in 62 μL of saline via a motorized pump. During each infusion, lever presses were recorded but did not result in further infusions. For animals trained with cocaine-associated cues, cocaine infusions were paired with discrete light+tone cues (white stimulus light above the active lever; 78 dB, 2900 Hz tone). The red house light on the wall opposite the levers was turned off during cocaine infusions. For animals trained without cocaine-associated cues, the only event that occurred with a lever press was pump activation and cocaine delivery. While the infusion pump was on, presses on the active lever were recorded but did not elicit a second infusion, and a new infusion could be initiated as soon as the previous infusion was completed; there was no “timeout” period. Presses on an inactive lever had no programmed consequences but were recorded. Rats remained on the FR-1 schedule for a minimum of 5 days and until they achieved at least 10 infusions in a session. Rats were then switched to the threshold procedure for training and testing.
The day after completion of FR-1 training, rats were trained on the within-session threshold procedure (Oleson et al., 2011) as previously reported (Bentzley et al., 2013). In this 110-min paradigm rats received access to decreasing doses of cocaine in successive 10-min intervals on a quarter logarithmic scale (383.5, 215.6, 121.3, 68.2, 38.3, 21.6, 12.1, 6.8, 3.8, 2.2, 1.2 μg per infusion) by decreasing pump infusion duration. The pump rate infusion times (8175, 4597, 2585, 1454, 818, 460, 259, 145, 82, 46, 26 ms) were based on an averaged measurement of the flow rates (1031.9 ± 2.2 μL/min) of 16 individual PMH100 pumps (Med-Associates, St Albans, VT, USA) with a 10 ml syringe (BD, Franklin Lakes, NJ, USA). For the cue version of the procedure, during an infusion while the pump was on the house-light was turned off and the light+tone cues were presented (similarly to the FR-1 paradigm). Light+tone and houselight events were omitted in the no-cue version of the procedure. While the pump was on active lever presses were recorded but did not elicit a second infusion. The animals were run daily on the threshold procedure for a minimum of 6 sessions and until the last 3 sessions produced an α value (described below) that was within a range of ±25% of the mean of those days. Baseline demand measures were taken as the mean of the last 3 days. After initial testing animals were re-stabilized on the threshold procedure for a minimum of 3 sessions and until the last 3 sessions produced an α value that was within a range of ±25% of the mean of those days.
Demand curve fitting
Demand curves were fit via our focused-fitting approach (Bentzley et al., 2013). Briefly, each animal’s brain cocaine concentration was calculated to determine relative stability during a session. Demand data points that failed to meet previously reported stability criteria were truncated before demand curves were fit by standard techniques. This typically resulted in elimination of the first data point, during which the subject ‘loaded’ on cocaine (Oleson et al., 2011), and data points that occurred after 2 points following the maximum price at which the subject maintained its preferred level of drug consumption (Pmax), when brain cocaine concentration had dropped significantly enough to provoke increased responding (Bentzley et al., 2013). Demand curves were fit to each subject’s truncated data set using standard regression techniques. In this approach, the values α and Q0 in the exponential demand equation (Hursh & Silberberg, 2008) were manipulated to minimize the residual sum of squares, i.e. the square of the difference between the logarithm of the experimentally measured demand and the logarithm of the demand predicted by the exponential demand equation was found for each price and then summed across all prices. α quantifies demand elasticity, an inverse measure of motivation for drug, and Q0 estimates consumption at null effort (Hursh & Silberberg, 2008). The parameter k represents the range of the consumption data in Loge units and was held constant at a value of 7.368 (3.2 in Log10 units) across all animals (Hursh & Silberberg, 2008). This value of k was chosen based on the maximum observed range of consumption.
Extinction and reinstatement procedures
Animals were run on daily extinction sessions for a minimum of 7 days and until the last 2 consecutive days had ≤ 25 responses on the active lever, i.e., the lever that previously activated a cocaine infusion. Extinction sessions were identical to self-administration sessions except that responses on the active lever had no consequence, and they were 120 min in duration. After animals reached extinction criteria, reinstatement of responding was tested with cocaine-associated light+tone cues. During cue-induced reinstatement testing, the same discrete light and tone cues that had been paired with cocaine infusions during acquisition and initial threshold testing were presented without cocaine, once at the start of the session and in response to active lever presses. Cues were 3.6s in duration and could only be elicited once every 20s; however, all active responses were recorded. In between reinstatement tests, animals were run until 2 consecutive days had ≤ 25 responses on the active lever.
Experimental design
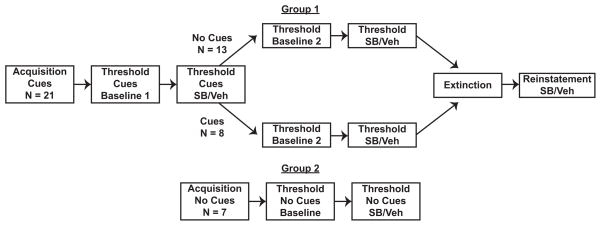
An overview of the experimental design can be found in Fig. 1. Group 1 animals (n = 21) were trained to self-administer cocaine with cocaine-associated light+tone cues and then trained on the within-session threshold procedure until stable α values were achieved. Animals were then pretreated with either SB or vehicle 30 min prior to testing on the within-session threshold procedure with light+tone cues in a within-subjects crossover design with 24 hrs between tests. Animals were then split into 2 subgroups to determine the role of cues in the magnitude of demand for cocaine and to determine if the effect of SB was dependent on cocaine-associate cues. One subgroup was re-stabilized on the standard threshold procedure with light+tone cues (n = 8) and the other subgroup without light+tone cues (n = 13). Subgroups were again pretreated with either SB or vehicle 30 min prior to testing on the within-session threshold procedure in a within-subjects crossover design with 24 hrs between tests. Animals were then run on extinction sessions and tested for cue-induced reinstatement with SB or vehicle pretreatment in a within-subjects crossover design.
Fig. 1.
Experimental design overview. Group 1 animals (n = 21) were trained to self-administer cocaine with light+tone cues and then trained on the within-session threshold procedure with light+tone cues. These animals were then pretreated with either SB or vehicle (veh) prior to testing on the within-session threshold procedure with cues. Group 1 animals were then split into 2 subgroups and re-stabilized on the threshold procedure either with (n = 8) or without light+tone cues (n = 13). Animals were again pretreated with either SB or vehicle prior to testing on the within-session threshold procedure. All Group 1 animals were then run on extinction sessions and tested for cue-induced reinstatement with SB or vehicle pretreatment in a counterbalanced fashion. Group 2 animals were trained to self-administer cocaine without light+tone cues and then trained and tested on the within-session threshold procedure without light+tone cues.
A control group of animals (Group 2, n = 7) was trained to self-administer cocaine without cocaine-associated light+tone cues and then trained on the within-session threshold procedure without light+tone cues until animals produced stable α values. Group 2 animals were then pretreated with either SB or vehicle 30 min prior to testing on the within-session threshold procedure without light+tone cues in a within-subjects crossover design with 24 hrs between tests.
Statistics
All statistics were performed using GraphPad Prism (Version 5.01) except multiple linear regression analyses were performed using SPSS Statistics (Version 19). Q0, α and reinstatement data were found to be positively skewed (Shapiro-Wilk, P < 0.05), sample sizes were relatively small in some cases (e.g., n = 7), and the coefficient of variation was found to be highly variable for some comparisons (e.g., >100% difference). Based on these considerations, non-parametric Wilcoxon signed-rank tests were used to determine if median changes in demand parameters were significant. Logarithmic transformation of Q0 and α variables produced normal distributions of each (Shapiro-Wilk, P > 0.05) for use in multiple linear regression analyses, as this type of analysis requires data to be normally distributed. For multiple linear regression analyses, Log10(Q0) and Log10(α) were set as the independent variables, and the dependent variable was set as either the total number of active lever responses during cued reinstatement after vehicle pretreatment or as the difference in responding between treatments, calculated as the number of active lever responses during cued reinstatement after SB pretreatment subtracted from the number of active lever responses during cued reinstatement after vehicle pretreatment. The proportional change in demand elasticity from baseline with SB treatment was calculated as [Log(αSB) − Log(αbaseline)]/Log(αbaseline) (Bentzley et al., 2014). This proportional change in demand elasticity was then related to the logarithmically transformed baseline demand elasticity, Log(αbaseline), via a Pearson correlation.
Results
SB pretreatment increases demand elasticity
Group 1 (Fig. 1) rats trained with cocaine-associated light+tone cues (n = 21) met the FR-1 acquisition criterion with means (±standard error of the mean) for the last 2 days of FR-1 cocaine self-administration of 19.6±1.9 and 21.6 ±1.3 infusions and 21.6 ±2.0 and 24.6 ±1.6 active lever presses (Fig. 2). Rats were then trained and stabilized on the within-session threshold procedure with light+tone cues for a mean of 11.7 ±1.1 days before SB or vehicle treatment. SB pretreatment significantly increased demand elasticity (α, an inverse measure of motivation) compared to vehicle pretreatment (Wilcoxon signed-rank, W = −121, P < 0.05) (Fig. 3A). In contrast, SB pretreatment had no effect on free cocaine consumption (Q0: Wilcoxon signed-rank, W = 67, P = 0.25) (Fig. 3B). Composite demand curves constructed from mean α and Q0 values demonstrate the increase in price-sensitivity with SB treatment (Fig. 3C). Finally, baseline demand elasticity was not correlated with the magnitude of change in elasticity following SB pretreatment (Pearson, r = 0.11, P = 0.65) (Fig. 3D).
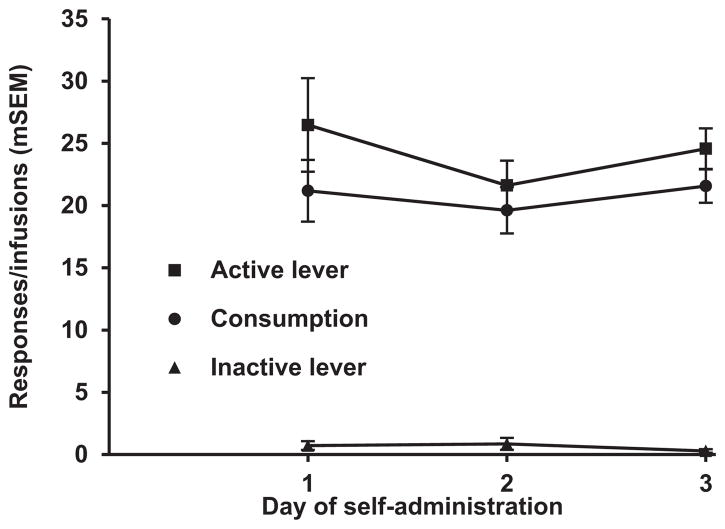
Fig. 2.
Behavior during cocaine self-administration under an FR-1 schedule of reinforcement with light+tone cues for the 3 days before training and testing on the within-session threshold procedure (n = 21, Group 1).
Fig. 3.
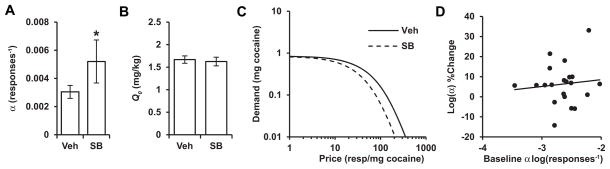
SB pretreatment increased cocaine demand elasticity without altering free cocaine consumption. (A) Normalized demand elasticity (α) was significantly higher in animals when they were pretreated with SB compared to when they were pretreated with vehicle (veh) (*P < 0.05). (B) Free cocaine consumption (Q0) was not altered by SB pretreatment compared to vehicle pretreatment. (C) Demand curves constructed from mean α and Q0 values to demonstrate the increased sensitivity of demand to price with SB treatment. (D) Baseline demand elasticity (abscissa) was not associated with change in demand elasticity from baseline with SB pretreatment (ordinate).
Removal of cocaine-associated light+tone cues increases demand elasticity
After the above tests of the effects of SB on demand, animals were run in the threshold demand procedure (without SB or Veh treatment injections) until they re-established baseline stability criteria. A portion of animals (n = 8) was re-stabilized on the standard threshold procedure with response-contingent light+tone cues, and remaining animals (n = 13) were re-stabilized on this same procedure but without cues. Compared to baseline demand with cues, median demand elasticity (α) remained unchanged in the group that was re-stabilized with discrete light+tone cues, although 2 animals showed a notable increase in demand elasticity (Wilcoxon signed-rank, W = −10, P = 0.55) (Fig. 4A). However, median α significantly increased in the group of animals that was re-stabilized without cues (Wilcoxon signed-rank, W = −50, P < 0.05) (Fig. 4B). In contrast, both groups showed a similar magnitude of free consumption increase (Q0; median increase ~0.5 mg/kg). This increase in Q0 approached significance in the group re-stabilized with cues (Wilcoxon signed-rank, W = −24, P = 0.11) (Fig. 4C) and reached significance in the group re-stabilized without cues (Wilcoxon signed-rank, W = −58, P < 0.01) (Fig. 4D).
Fig. 4.
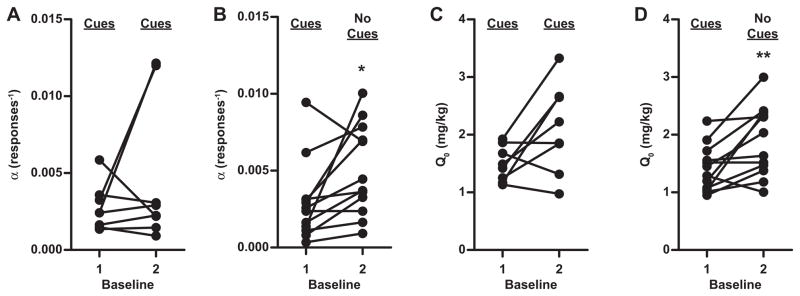
Removal of response-contingent, cocaine-associated light+tone cues led to an increase in demand elasticity. Each line represents data from an individual subject. All animals received cues during their training and during their first baseline demand test. (A) Animals that were retested for baseline demand with cues showed no overall change in baseline demand from the initial test, i.e., median α remained unchanged, although 2 animals showed a notable increase in demand elasticity. (B) Animals that were retested for baseline demand without cues showed a significant increase in median demand elasticity (α) (*P < 0.05). (C & D) Both groups showed a similar magnitude increase in median free cocaine consumption (Q0) during retesting (~0.5 mg/kg increase). This increase did not reach significance in the group retested with cues (C) but was significant in the group retested without cues (D; **P < 0.01).
The effect of SB on demand elasticity depends on cocaine-associated cues
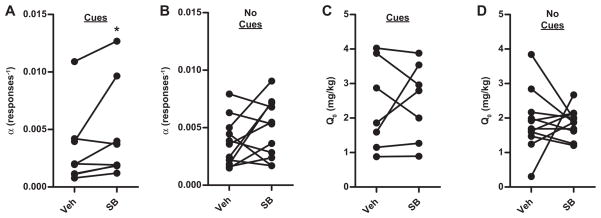
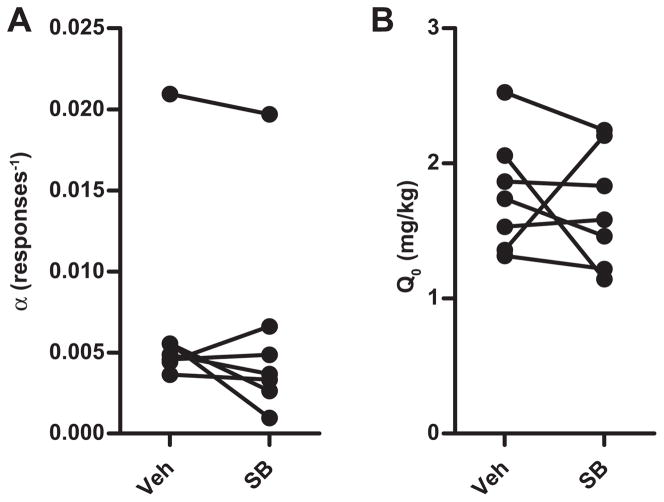
SB pretreatment significantly increased demand elasticity (α) in animals re-stabilized and tested with light+tone cues (Wilcoxon signed-rank, W = −28, P = 0.05) (Fig. 5A), but SB pretreatment did not significantly change elasticity in animals re-stabilized and tested without light+tone cues (Wilcoxon signed-rank, W = −24, P = 0.32) (Fig. 5B). Free cocaine consumption, Q0, was unaltered with SB pretreatment in either group (Fig. 5C; with cues: Wilcoxon signed-rank, W = −4, P = 0.81) (Fig. 5D; without cues: Wilcoxon signed-rank, W = 8, P = 0.76). Furthermore, in a separate group of animals (n = 7) trained and tested without exposure to light+tone cues (Fig. 1, Group 2), SB pretreatment did not alter demand elasticity (Wilcoxon signed-rank, W = 16, P = 0.22) (Fig. 6A) or free cocaine consumption (Wilcoxon signed-rank, W = 12, P = 0.38) (Fig. 6B) compared to vehicle pretreatment.
Fig. 5.
SB pretreatment significantly increased cocaine demand elasticity only when response-contingent, cocaine-associated light+tone cues were present. (A) In the group re-stabilized and retested with light+tone cues, SB pretreatment significantly increased demand elasticity (α) (*P = 0.05). (B) In contrast, SB pretreatment did not increase demand elasticity in the group re-stabilized and retested without light+tone cues. (C and D) Free cocaine consumption (Q0) did not change as a function of SB treatment when cues were present (C) nor when cues were absent (D).
Fig. 6.
(A) SB pretreatment did not increase cocaine demand elasticity (α) when animals were trained and tested without response-contingent, cocaine-associated light+tone cues. (B) SB pretreatment did not alter free cocaine consumption (Q0) in this control group of animals trained and tested without cocaine-associated cues.
SB attenuates cue-induced reinstatement of cocaine seeking in proportion to baseline demand
Next, animals were run on daily extinction sessions until responding on the previously active lever decreased to ≤ 25 responses for 2 consecutive days. Animals were then pretreated with SB or vehicle before cue-induced reinstatement testing in a counterbalanced, within-subjects manner. SB pretreatment significantly reduced active lever responding during cue-induced reinstatement from 63.8 (±10.3) responses after vehicle pretreatment to 19.9 (±3.5) responses after SB (Wilcoxon signed-rank, W = 228, P < 0.001). Multiple linear regression analysis revealed that the magnitude of responding during cued reinstatement after vehicle pretreatment was predicted by baseline demand elasticity (α: β = −0.69, P < 0.01) but not by baseline free consumption (Q0: β = 0.05, P = 0.81) (Fig. 7A) with animals with the lowest α (highest motivation) responding the most during reinstatement testing. In addition, the reduction in responding during cue-induced reinstatement following SB was predicted by animals’ baseline cocaine demand elasticity (α) taken before any SB treatment (β = −0.65, P < 0.01) but not by Q0 (β = 0.02, P = 0.93) (Fig. 7B).
Fig. 7.
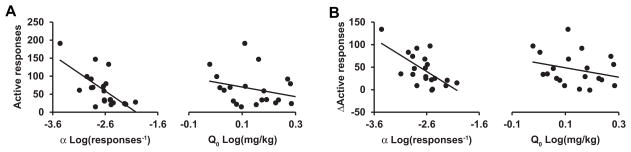
Baseline demand predicted reinstatement propensity and efficacy of SB treatment. (A) Baseline demand elasticity (α), but not free cocaine consumption (Q0), significantly predicted the magnitude of cue-induced reinstatement with vehicle pretreatment. (B) Baseline demand elasticity (α), but not free cocaine consumption (Q0), significantly predicted the magnitude of the reduction of cue-induced reinstatement with SB pretreatment.
Discussion
Here, we showed that systemic blockade of OX1Rs with SB increased the elasticity of demand for cocaine (α; an inverse measure of motivation) but did not alter free cocaine consumption (Q0). Removal of cocaine-associated, response-contingent light+tone cues also reduced rats’ motivation for cocaine (increased α), and the effect of OX1R antagonism on demand elasticity was dependent on the presence of these cues. After responding on the active lever was extinguished, pretreatment with SB blocked cue-induced reinstatement of cocaine seeking. Finally, we showed that rats’ baseline cocaine demand elasticity, measured before any testing, predicted later cue-induced reinstatement propensity as well as the effect of OX1R antagonism in reducing this propensity. Taken together these results indicate that OX1R signaling underlies the reinforcing efficacy of cocaine-associated cues but not the reinforcing efficacy of cocaine alone.
OX1R function in cocaine demand depends on cocaine-associated cues
We found that cocaine demand elasticity was significantly lower when response-contingent, cocaine-associated light+tone cues were present. This increased drug demand when drug-associated stimuli are present has been found in human subjects exposed to alcohol-associated (MacKillop et al., 2010) or nicotine-associated cues (MacKillop et al., 2012; Acker & MacKillop, 2013). We extended these studies by showing that this effect of cues on demand is also present in animals and for cocaine. This increase in demand in response to drug cues is also supported by evidence that rats prone to attributing incentive salience to cues (i.e., “sign trackers”) show high motivation for cocaine (Saunders & Robinson, 2011), and that cocaine-associated cues are sufficient to maintain responding in the absence of drug delivery (Di Ciano & Everitt, 2004). This line of research has led to the conclusion that such drug-associated cues can take on motivational properties that drive behavior independently of the presence of the unconditioned drug reinforcer (Robinson et al., 2014). We hypothesize that the presence of response-contingent cues augments the motivational properties of cocaine, especially at high prices when the magnitude of cocaine delivery is small such that a larger portion of the total reward is contributed by concomitant delivery of drug-associated cues.
We found that SB pretreatment resulted in significantly increased demand elasticity only when cocaine delivery was paired with light+tone cues, and this effect did not depend on whether cues were present during self-administration training or on baseline cocaine demand. This effect is consistent with prior reports that SB pretreatment blocked reinstatement of extinguished cocaine seeking in response to cocaine-associated cues but not in response to a passive drug exposure (Smith et al., 2009; Mahler et al., 2013). Thus, orexin signaling is needed for the motivational efficacy of cocaine-associated cues but not for cocaine itself (Smith et al., 2009).
Notably, the effect of SB on cocaine demand that we report here was only present at high cocaine prices, an effect also documented in a previous study (España et al., 2010), and in the threshold procedure employed, high prices are associated with smaller cocaine doses. Thus, at high prices cocaine-associated cues contribute a larger proportion to the motivational efficacy of the delivered reward; we conclude that this may increase the sensitivity of responding at high prices to OX1R blockade. This hypothesis in inline with previous studies that found that OX1R signaling in VTA potentiates glutamatergic responses of dopamine neurons (Borgland et al., 2009; Moorman & Aston-Jones, 2010), and this potentiation is necessary for cued-induced, but not cocaine-primed, reinstatement of cocaine seeking (Mahler et al., 2013). Thus, we hypothesize that orexin-augmented glutamatergic signaling in VTA contributes to the increase in demand (motivation) with cocaine-associated light+tone cues.
We found that OX1R antagonism increases normalized demand elasticity for cocaine. This measure of normalized elasticity (α) is thought to capture the motivational quality of a reward and is also known as ‘essential value’ (Hursh & Silberberg, 2008). Thus, our findings confirm that orexin signaling is necessary for motivation to obtain cocaine, as has been previously suggested (Thompson & Borgland, 2011; Mahler et al., 2012) and shown using non-normalized demand elasticity (España et al., 2010). Normalized measures of elasticity are often associated with clinical addiction severity (Bentzley et al., 2013), and we recently found that normalized demand elasticity predicts a broad spectrum of addiction-like behavior in the rat independently of free cocaine consumption, and free cocaine consumption rarely adds to the predictive capacity of these models (Bentzley et al., 2014). Thus, testing with normalized measures of demand elasticity increases confidence in predictions of clinical treatment potential compared to testing with non-normalized measures. Our finding here that SB treatment increased the normalized elasticity parameter, α, indicates that OX1R antagonists have clinical potential for treatment of psychostimulant use disorders.
OX1R signaling links demand and cued reinstatement
A major goal of addiction therapies is to attenuate the high propensity to drug relapse (Kalivas & O’Brien, 2008). We recently showed that economic demand for cocaine provides a continuous scale of addiction severity that predicts later relapse propensity in rats (Bentzley et al., 2014). Other reports have indicated a similar association between demand and relapse for methamphetamine (Galuska et al., 2011), and we have replicated our results for cocaine in this report by showing that baseline demand predicted later cued reinstatement after vehicle pretreatment. Given our hypothesis that OX1R signaling increases motivation for cocaine by augmenting cue-associated glutamate responses of dopamine neurons (Moorman & Aston-Jones, 2010; Mahler et al., 2012), we predicted that OX1R antagonism would preferentially reduce cued-reinstatement in animals with high baseline demand for cocaine, i.e., animals with low demand elasticity that are insensitive to drug devaluation. Stated otherwise, animals with the lowest demand elasticity are the animals that reinstate the most in response to drug cues, and it is these animals that we predict would be the most sensitive to therapies that target the reinforcing efficacy of drug cues. Indeed, we found that baseline demand elasticity predicted the reduction in cued-reinstatement with SB; this adds another example of how drug demand can predict treatment outcomes (MacKillop & Murphy, 2007; Bentzley et al., 2014). This finding also highlights the clinical appeal of OX1R antagonism in that it selectively targets excessive motivation/demand inelasticity, an important element in addiction. This also adds credence to the hypothesis that demand can be used to test the clinical potential of prospective addiction treatments (Bentzley et al., 2014).
Conclusions
Systemic OX1R antagonism with SB significantly increases demand elasticity when response-contingent, light+tone cues are present. In contrast, SB treatment does not alter free cocaine consumption. Taken together these results indicate that OX1R signaling underlies the reinforcing efficacy of cocaine-associated cues but not of cocaine alone. In concordance with this conclusion, cocaine demand elasticity provided a robust prediction of the magnitude of later reductions in cue-induced relapse propensity by an OX1R antagonist, with the largest effects in animals that were high-responders in both cue-dependent tasks. Orexin-based therapies hold potential as clinical treatments for cocaine addiction by selectively inhibiting the augmenting effects of cocaine-associated cues on cocaine taking and seeking.
Acknowledgments
We thank Kimberly M. Fender for her indispensable assistance with behavioral testing. This work was supported by U.S. Public Health Service grant awards R37/R01-DA006214, T32 GM008716, and F30-DA035065.
Abbreviations
- FR
fixed-ratio
- OX1R
orexin 1 receptor
- SB
SB-334867
References
- Acker J, MacKillop J. Behavioral economic analysis of cue-elicited craving for tobacco: a virtual reality study. Nicotine Tob Res. 2013;15:1409–1416. doi: 10.1093/ntr/nts341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G. The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology. 2013;226:113–125. doi: 10.1007/s00213-012-2899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proceedings of the National Academy of Sciences. 2014;111:11822–11827. doi: 10.1073/pnas.1406324111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. Journal of Neuroscience. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Piat F, Le Moal M, Piazza PV. Influence of cue-conditioning on acquisition, maintenance and relapse of cocaine intravenous self-administration. Eur J Neurosci. 2002;15:1363–1370. doi: 10.1046/j.1460-9568.2002.01974.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47(Suppl 1):202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- España RA, Melchior JR, Roberts DCS, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology. 2011;214:415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DCS, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galuska CM, Banna KM, Willse LV, Yahyavi-Firouz-Abadi N, See RE. A comparison of economic demand and conditioned-cued reinstatement of methamphetamine-seeking or food-seeking in rats. Behavioural Pharmacology. 2011;22:312–323. doi: 10.1097/FBP.0b013e3283473be4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Winger G. Normalized demand for drugs and other reinforcers. J Exp Anal Behav. 1995;64:373–384. doi: 10.1901/jeab.1995.64-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, Dayas CV. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharm. 2011;14:684–690. doi: 10.1017/S1461145711000423. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropschopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Murphy JG. A behavioral economic measure of demand for alcohol predicts brief intervention outcomes. Drug Alcohol Depend. 2007;89:227–233. doi: 10.1016/j.drugalcdep.2007.01.002. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Brown CL, Stojek MK, Murphy CM, Sweet L, Niaura RS. Behavioral economic analysis of withdrawal- and cue-elicited craving for tobacco: an initial investigation. Nicotine Tob Res. 2012;14:1426–1434. doi: 10.1093/ntr/nts006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, O’Hagen S, Lisman SA, Murphy JG, Ray LA, Tidey JW, McGeary JE, Monti PM. Behavioral economic analysis of cue-elicited craving for alcohol. Addiction. 2010;105:1599–1607. doi: 10.1111/j.1360-0443.2010.03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2013;226:687–698. doi: 10.1007/s00213-012-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences. Journal of Neuroscience. 2010;30:15585–15599. doi: 10.1523/JNEUROSCI.2871-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Richardson JM, Roberts DCS. A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology. 2011;214:567–577. doi: 10.1007/s00213-010-2058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RA, Chan WN, Coulton S, Johns A, Hadley MS, Widdowson K, Jerman JC, Brough SJ, Coldwell M, Smart D, Jewitt F, Jeffrey P, Austin N. 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg Med Chem Lett. 2001;11:1907–1910. doi: 10.1016/s0960-894x(01)00343-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76(Pt B):450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones GS. A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. Journal of Neuroscience. 2012;32:4623–4631. doi: 10.1523/JNEUROSCI.4561-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropschopharmacology. 2011;36:1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, Jerman JC. SB-334867-A: the first selective orexin-1 receptor antagonist. Br J Pharmacol. 2001;132:1179–1182. doi: 10.1038/sj.bjp.0703953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58:179–184. doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JL, Borgland SL. A role for hypocretin/orexin in motivation. Behavioural Brain Research. 2011;217:446–453. doi: 10.1016/j.bbr.2010.09.028. [DOI] [PubMed] [Google Scholar]