Abstract
Scope
Acute kidney injury (AKI) is the most frequent and serious complication in sepsis, a potentially deadly inflammatory response induced by bacterial, viral or fungal infection. Lipopolysaccharide (LPS) induced AKI is associated with an abnormal inflammatory response, including renal endothelial dysfunction and renal inflammation. Resveratrol, a natural phytoalexin with low toxicity and anti-inflammatory properties, is known to protect endothelial cells and modulate the immune response in sepsis.
Methods and results
This study investigates the potential protective effects of resveratrol on AKI induced by LPS exposure of mice. Resveratrol was administered as a pre- and post-treatment, or as a post-treatment alone following LPS injection and compared to control groups.
Resveratrol significantly improved kidney function and lowered serum and kidney tissue inflammatory cytokine levels. Consistently, resveratrol prevented endotoxin-induced disruption of endothelial cell permeability and inhibited inflammation of kidney tissue. Resveratrol treatment attenuated the effects of LPS on macrophages, with significant inhibition of activation, cytokine release and TLR4 activation. Resveratrol treatment also resulted in decreased expression of iNOS, Bcl-2 and Bcl-xL in macrophages, which was linked with induction of apoptosis in macrophages.
Conclusion
Our studies suggest that resveratrol might represent a novel therapeutic agent to prevent and treat sepsis induced AKI.
Keywords: resveratrol, LPS, kidney injury, macrophage, AKI
Introduction
Sepsis is a complex clinical condition characterized by a whole-body inflammatory response that results from a harmful host response to infection [1]. Acute kidney injury (AKI) is one of the most frequent ramifications of sepsis and increases both the complexity of the condition and the mortality rate compared with sepsis alone [2]. Unfortunately, the exact pathophysiology of AKI in sepsis still poorly understood. To date, the main clinical therapies for AKI, such as fluid resuscitation and supportive care, emphasize the role of renal blood flow (RBF) to maintain kidney perfusion. These treatments have improved survival, however the mortality rates of AKI remain unacceptably high at 30% [3, 4]. Even animal models of sepsis have demonstrated little improvement following marked elevation of RBF in sepsis related AKI [5]. These might indicate that maintenance of hemodynamic stability may not be sufficient for AKI treatment, with nonhemodynamic conditions, such as immunologic or inflammatory factors, also contributing to development of septic AKI [6–8].
Lipopolysaccharide (LPS), produced by gram-negative bacteria, has been widely used for AKI research [9]. The AKI model induced by LPS results in an immune response that initiates kidney injury. Mononuclear and endothelial cells play a role in the initial immune response [1]. After exposure to LPS, resident renal mononuclear cells secrete chemokines and cytokines that guide the immune cell from the peripheral circulation into the inflamed site to fight infection. Endothelial cells are also involved in this process with regard to regulation of vascular permeability [10, 11]. Among resident and infiltrating mononuclear cells, macrophages play a critical role in the pathology of AKI. Depleting macrophages or reducing macrophage activation protects the kidney from injury [12, 13]. During endotoxemia, LPS-induced Toll-like receptor-4 (TLR-4) activation in macrophages triggers transcription of nuclear factor-κB (NF-κB), a pro-inflammatory gene that induces a rapid cytokine storm, including spikes in TNFα, IL-1β and IL-6. [14, 15]. These cytokines, in turn, activate a large number of neutrophils, macrophages and dendritic cells. These activated immune cells produce reactive oxidative species (ROS), neutrophil elastase, inflammatory factors and damage the surrounding tissue [16, 17]. Therefore controlling abnormal immune responses in the kidney might prevent AKI and improve the clinical outlook in sepsis.
Resveratrol (3, 5, 4-trihydroxistilbene), a phytoalexin found in many plants with anti-inflammatory, anti-tumor, and anti-oxidant properties, has been widely studied in various animal models for human disease [18, 19]. Resveratrol has low renal toxicity and is known to have beneficial effects in renal disease, including sepsis-induced renal injury [20, 21]. Many molecular pathways are involved in the anti-inflammatory effects of resveratrol, including TLR4 and NF-κB pathways. For example, Resveratrol suppresses TLR4 activation and decreases cytokine production in LPS stimulated macrophages [22, 23]. In this study, we investigated the mechanism behind the local immune response in AKI caused by LPS infusion. We found that resveratrol mediates anti-inflammatory responses by several pathways, and thereby offers a potential therapeutic strategy for AKI.
Materials and Methods
Animals
Female C57BL/6 mice aged 8 to 12 weeks were purchased from the National Cancer Institute. Animals were housed in the animal facility at the University of South Carolina School of Medicine and maintained under normal light and dark cycles. Animal use procedures were approved by the Institutional Animal Care and Use Committee of the University of South Carolina (Protocol AUP 2169).
In vivo experiments
For in vivo experiments, 8–10 week old female C57BL/6 mice (n=8 per group) were injected intraperitoneally with 15 mg/kg body weight of LPS (Escherichia coli O111:B4, Sigma-Aldrich) in 100 µl pyrogen-free water. The control group received 100 µl of pyrogen-free water. To evaluate the anti-inflammatory effect of resveratrol, two separate groups were treated with resveratrol, a pre-LPS treatment group and a post-LPS treatment group. Mice received 100 µl of 100 mg/kg of resveratrol (Sigma-Aldrich) suspended in 100 µl distilled water by oral gavage. In the pretreatment group (RES+LPS), resveratrol was administered 6 h before and 2 h after LPS injection. In the post-treatment group (LPS+RES), resveratrol was administered 2 h after LPS injection. At 20 h after the injection of LPS or control buffer, mice were euthanized. Blood was collected in heparinized tubes. Both kidneys were removed with one kidney from each mouse was kept at −80°C freezer and the other kidney fixed in 10% formalin.
Renal function and cytokine analysis
For assessing renal function, blood urea nitrogen (BUN) and serum creatinine were measured with ELISA kits (Sigma) according to the manufacturer’s instructions. Blood samples were taken from the retro-orbital plexus, and plasma was isolated. Frozen kidney tissues were homogenized in lysis buffer (150 mM NaCl, 15 mM Tris, 1mM MgCl2 pH 7.4, 1 mM CaCl2, 1% Triton) with 1% protease inhibitor cocktail (P8340, Sigma). Cytokine concentration in plasma, kidney homogenates or cell supernatants were detected with an ELISA kit (Biolegend) for tumor necrosis factor (TNF)-a, interleukin (IL)-1β, IL-6, IL-10 and monocyte chemotactic protein (MCP-1). The procedures were performed according to the manufacturer’s protocol.
Histopathology and immunochemistry
Renal tissues were placed in 10% buffered formalin overnight and subsequently embedded in paraffin. Renal sections of 4 µm thickness were stained with haematoxylin and eosin. Vacuolar degeneration of kidney tubular cells was scored as follows: less than 5% = 0, 5% – 20% = 1+, 20%–50% = 2+, and more than 50% = 3+.
Evaluation of renal cell apoptosis was performed on 4 µm renal sections using the DeadEnd Colorimetric TUNEL System from Promega by following the manufacturer’s instructions. TUNEL positive stained renal cells were counted in 10 randomly selected fields for each slide and the data was expressed as the percentage of apoptotic cells per field.
Macrophages were isolated from inflammatory cells from kidney tissue using magnetic beads for F4/8 positive cells and evaluated for purity by FACS. Macrophages represented 94.2% of the positive cells. The cells were then placed on microscope slides and immersed in 4% paraformaldehyde in PBS and blocked with serum, then incubated with rabbit polyclonal anti-mouse iNOS antibody (Santa Cruz) at 4°C overnight. FITC-Avidin secondary antibody (Vector Laboratories) was used to detect the expression of iNOS in macrophages. Nuclei were counterstained with DAPI (Thermo Fisher Scientific). Positive cells were counted in 10 randomly selected fields from each slide and the data were expressed as the percentage of iNOS positive cells per field.
Vascular permeability assay by Evans blue dye
Twenty hours after LPS injection, mice from each group were injected with 1% Evans blue in PBS intravenously, and 2 h after Evans blue injection, the mice were euthanized. Mice were perfused with heparinized PBS, the kidneys were excised and homogenized in 1 ml formamide then placed at 37°C for 24 h. The amount of Evans Blue in the kidney was determined by measuring the absorbance of the supernatants at 620 nm. The following formula was used to calculate relative alteration in vascular leak: (ODsample − ODcontrol)/ODcontrol×100 [24].
Flow cytometry assay
To investigate inflammatory cells in kidney tissues, mice were treated with LPS and resveratrol as described above, kidney tissue was collected from euthanized mice 20 h after LPS injection. The kidney tissue was minced and digested in type IV collagenase (2 mg/ml) at 37 °C for 1 h. The digested kidneys were passed through 100- and 40-mm mesh, separated using two-phase Percoll (36% and 72%) and centrifuged at 1,000×g for 30 min at room temperature. Inflammatory cells were collected in the Percoll interface and washed in PBS containing 2% fetal bovine serum (FBS). After that, the cells were stained for 30 min at 4°C with F4/8 and GR-1 antibodies and analyzed with a Beckman 500 flow cytometer.
Western blot
Proteins were isolated from kidney tissues or cells with RIPA buffer containing protease inhibitors, PMSF, and sodium orthovanadate. Protein (50 µg) was separated by SDS-PAGE and transferred onto a PVDF membrane using a wet transfer apparatus. The blots were blocked with 5% non-fat dry milk in TBS. The blots were incubated with primary antibodies at 4°C overnight. The blots were washed and incubated with horseradish peroxidase labeled secondary antibodies at 4°C. After 2 h, protein was detected using Pierce ECL Western Blotting Substrate (Pierce).
In vitro experiments
Bone marrow cells were isolated from femurs of 8–10 week old female C57BL/6 mice. Cells were seeded in bacterial plastic plates for 4 h in RPMI 1640 medium containing 10% FBS. Non-adherent cells were removed and those remaining were cultured in the above medium containing 30% L929 cell-conditioned medium (L929 CM) as the source of murine macrophage colony-stimulating factor (M-CSF). After 7 days, cells were harvested and evaluated for purity by FACS staining with F4/80: 97.3% of the cells were positive. Bone marrow-derived macrophages (BMDM) were cultured in RPMI 1640 medium with 100 ng/ml LPS for 2 hours, then various concentrations of resveratrol (10, 20, 50, 100 µmol) were added into the medium. After incubating for an additional 12 h, BMDM cells were harvested and apoptosis detected using an Annexin V-FITC Apoptosis Kit (BD Biosciences).
Data analysis
Data were presented as mean ± standard error of three independent experiments. Statistical significance was performed using the ANOVA or Student's t test to evaluate differences between treated and control groups. P< 0.05 was considered to be significant.
Results
Resveratrol reduces cytokine levels and protects renal function in mice exposed to LPS
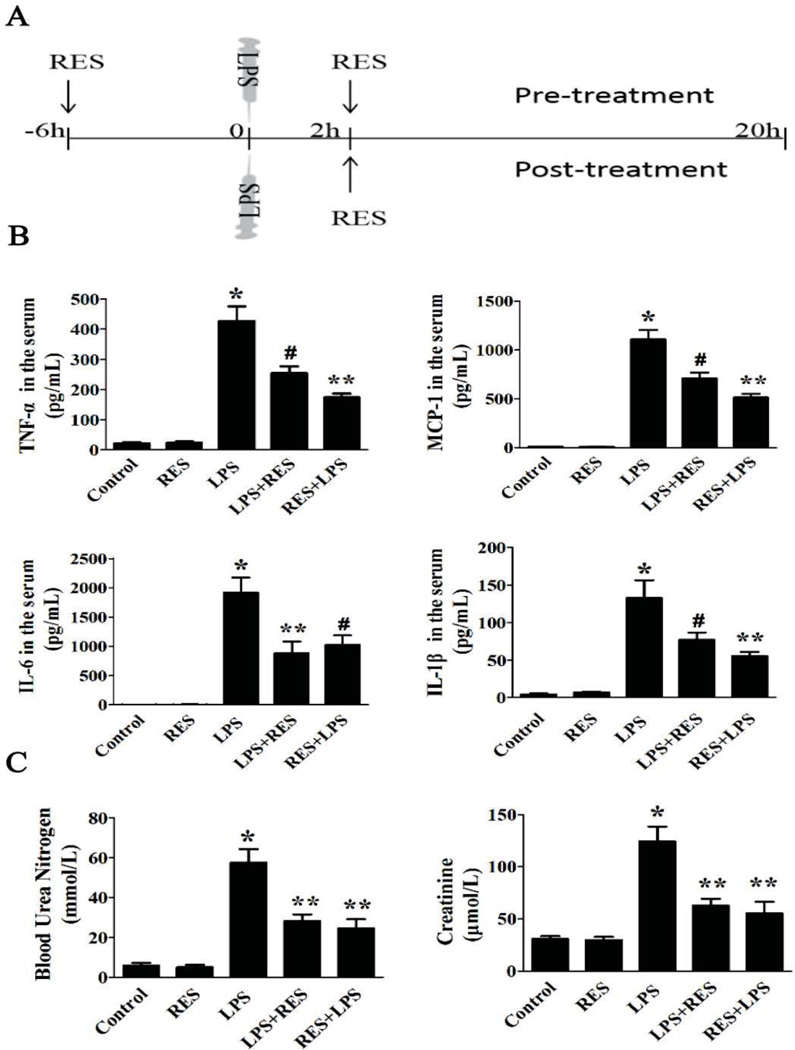
The goal of this study was to investigate whether resveratrol protects mice from the systemic inflammation and renal dysfunction induced by LPS [9]. To that end, resveratrol was administered in two different treatment regimens. In the pre-treatment group, mice received two oral doses of resveratrol: 6 h before LPS injection and 2 h after LPS injection. In the post-treatment group, mice only received a single dose: 2 h after LPS injection (Fig. 1A).
Figure 1. Resveratrol preserves renal function and reduces cytokine production induced by LPS.
A. Timeline of experimental design for the animal studies. LPS (15 mg/kg) or vehicle was given by intraperitoneal injection. The resveratrol was administered in two ways. In the pre-treatment group, mice received two oral doses of resveratrol: 6 h before LPS injection and 2 h after LPS injection. In the post-treatment, mice only received a single dose: 2 h after LPS injection. Mice were euthanized 20 h after LPS injection for evaluation. B. The concentration of IL-1β, IL-6, MCP-1 and TNFα in plasma of different groups was measured by ELISA. C. Serum BUN and creatinine were measured to assess renal function. Groups containing 8 mice each were as follows, LPS: LPS injected group; RES: Resveratrol only group; RES + LPS: LPS + Resveratrol pre-treatment group; LPS + RES: LPS + Resveratrol post-treatment group. Data were presented as mean ± SEM. (n=8, *p<0.01 vs. Control, **p<0.01 vs. LPS, #p<0.05 vs. LPS).
At 20h after LPS injection, serum from the mice in each group was collected for ELISA. The result (Fig. 1B) showed that exposure to LPS induced marked increase in cytokines, including TNFα, IL-1β, IL-6 and MCP-1, as well as significantly increased serum creatinine and BUN levels, which are a measure of kidney function, compared to the control group. In contrast, resveratrol pre-treatment and post-treatment caused a significant decrease in the levels each of these cytokines as well as creatinine and BUN (Fig. 1C). Interestingly, the pre-treatment group showed less of an effect on serum cytokine levels such as TNFα, IL-1β and MCP-1 and less of an effect on serum IL-6 levels compared with the post-treatment group. However, the renal function, as measured by serum creatinine and BUN levels, was not significantly different between pre-treatment and post-treatment groups.
Resveratrol ameliorates renal morphological changes and reduces infiltration of immune cells in the kidney
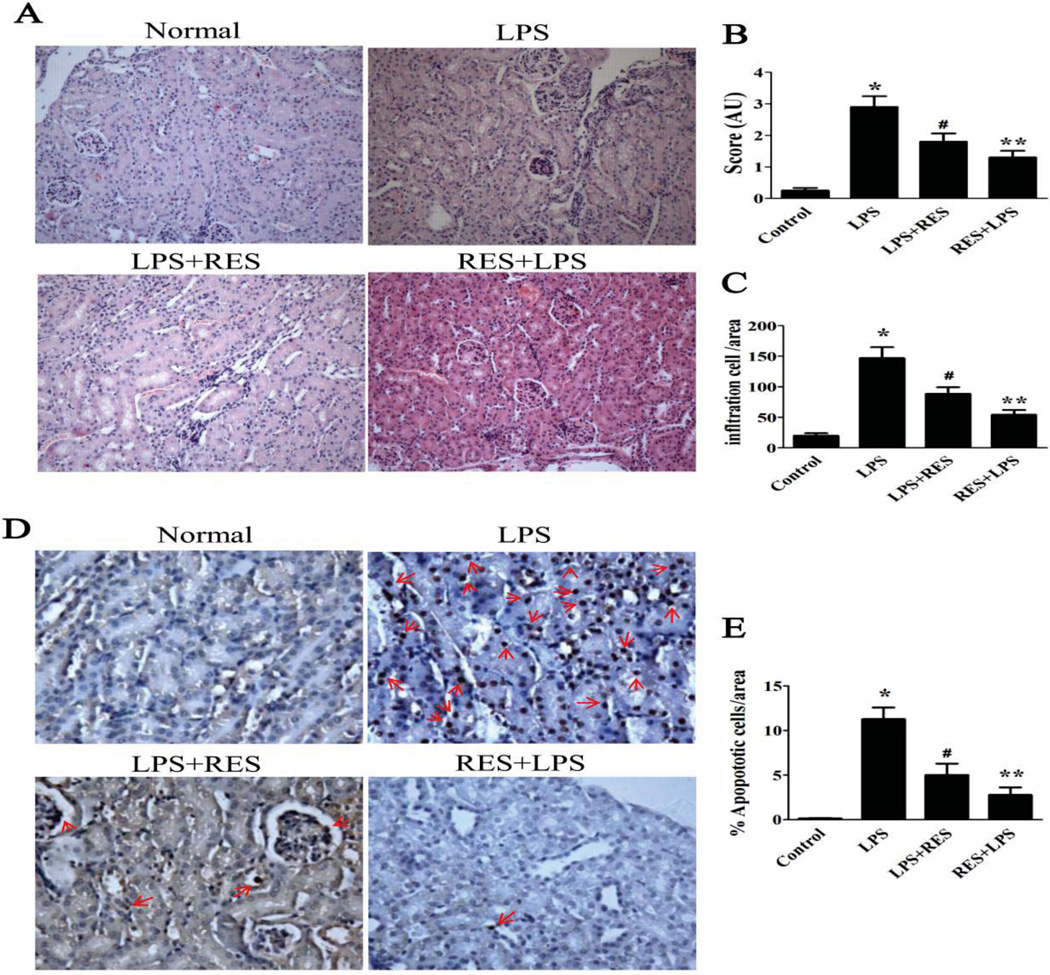
To further assess the protective effect of resveratrol on AKI, the kidneys of mice exposed to LPS were evaluated by histological analysis (Fig. 2). Renal tubular vacuolization and apoptosis are major pathologies observed during AKI [25]. Our data indicated that LPS induced significant renal tubular vacuolar changes (Fig. 2A–B) and renal tubular cell apoptosis (Fig. 2D--E), which were determined by assay for detecting TUNEL positive cells. Pre-treatment and post-treatment groups with resveratrol, displayed relatively mild renal morphological changes. Resveratrol decreased the LPS-induced tubular degeneration and the percentage of TUNEL-positive cells in the kidney. In contrast, the resveratrol alone group does not show any renal morphological changes compare to control group (data not shown). These data indicated that resveratrol has a protective effect against LPS-induced toxicity in kidney tissue.
Figure 2. Resveratrol ameliorates renal morphological changes and reduces infiltration of immune cells in the kidney.
A. Representative photographs of haematoxylin-eosin stained renal sections for each group of mice were taken at 200×. B. Renal tissue injury was quantitatively estimated in 10 random fields of the same size. C. Infltrating cells in renal tissue were quantitated in 10 random fields of the same size. D. Representative micrographs (200×) of TUNEL stained renal sections from different groups. E. The percentage of TUNEL-positive cells per kidney section in 10 random fields of the same size. The groups were designated as follows, LPS: LPS injected group; RES + LPS: LPS+Resveratrol pre-treatment group; LPS + RES: LPS + Resveratrol post-treatment group. AU: arbitrary unit; Data were presented as mean ± SEM. (n=8, *p<0.01 vs. Control, **p<0.01 vs. LPS, #p<0.05 vs. LPS).
Concurrently, we found that infiltration of inflammatory cells within the kidney from LPS-treated mice, which is associated with the pathology of AKI, with significantly increased levels inflammatory cells in the renal interstitium (Fig. 2 C). However, resveratrol treatment ameliorated the extent of infiltration, and the suppressive effect on infiltrating cells in the pre-treatment group was better than that in the post-treatment group. These results suggested that pre-treatment with resveratrol is more effective at reducing the inflammation in this model of murine AKI.
Resveratrol reduces infiltrating inflammatory cells and decreases renal endothelial permeability
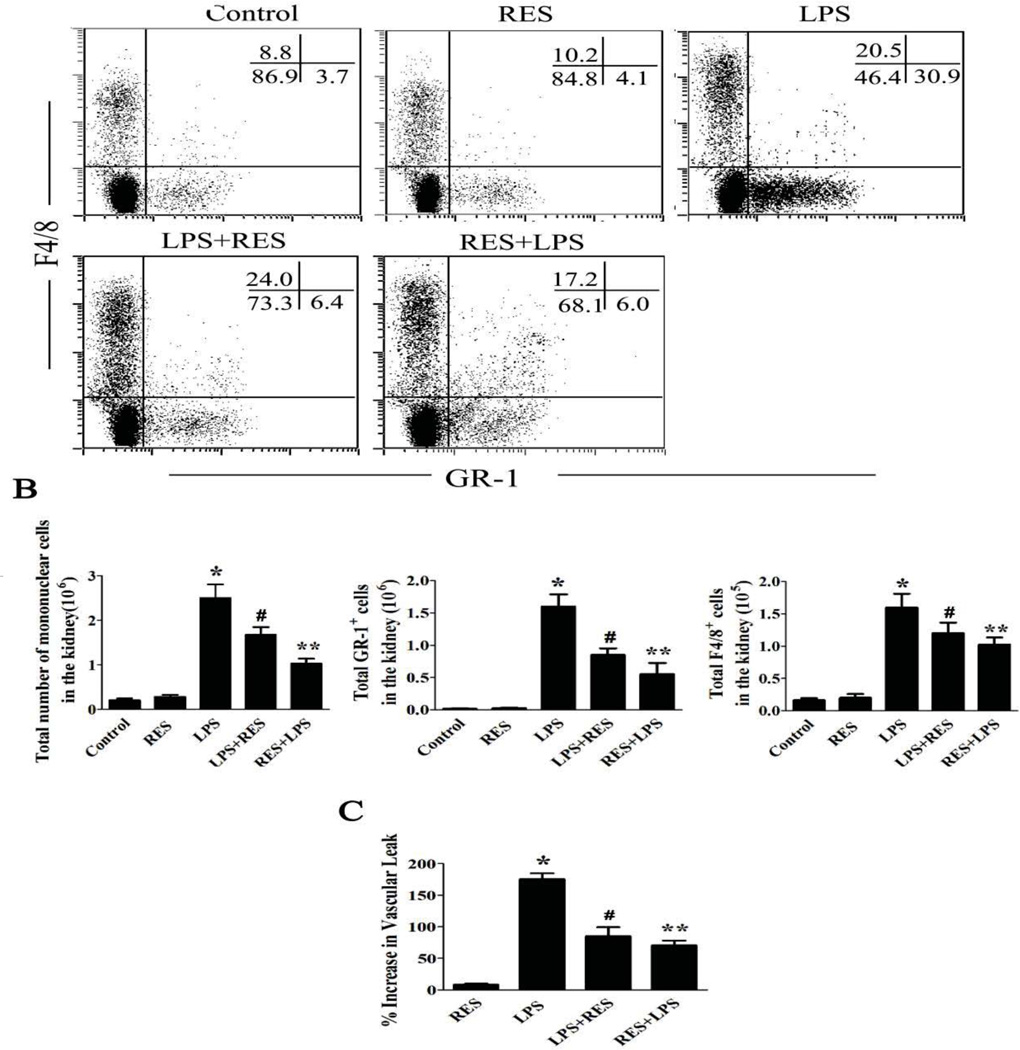
To investigate the local immune response within the kidney, the infiltrating cells within the kidney were isolated and stained with various cell markers. The percentage of cells and total inflammatory cell infiltrate in the different treatment groups were analyzed by flow cytometry (Fig. 3A). The total inflammatory cell infiltrate in the different treatment groups was consistent with the observations noted in the histological analysis. Administration of LPS significantly increased granulocytes and macrophages in the kidney (Fig. 3B). Resveratrol treatment significantly reduced the infiltrating inflammatory cells, particularly the granulocytes.
Figure 3. Resveratrol reduces infiltrating inflammatory cells and decreases renal endothelial permeability.
LPS, control buffer, and resveratrol were administered as described above. A. Representative dot-plots for phenotyping inflammatory cells in the kidneys. Mice were treated as above; renal infiltrative immune cells were isolated. Cells were stained with granulocyte (GR-1) and macrophage (F4/8) markers. B. The number of total cells, granulocytes and macrophages isolated from the kidney in each mouse are shown in the diagram, respectively. C. Renal vascular permeability was measured with Evans blue dye. The increased vascular permeability was shown in the bar diagram and compared to a control group. The groups were designated as follows, LPS: LPS injected group; RES: Resveratrol only group; RES + LPS: LPS + Resveratrol pre-treatment group; LPS + RES: LPS + Resveratrol post-treatment group. Data were presented as mean ± SEM. (n=8, *p<0.01 vs. Control, **p<0.01 vs. LPS, #p<0.05 vs. LPS).
To further investigate the mechanism by which resveratrol modulates the local immune response within the kidney, we assessed the function of the renal endothelium cells by assaying vascular permeability caused by infiltration of inflammatory cells into the kidney. To this end, we tested the effect of resveratrol on the kidney vascular permeability by measuring the leakage of Evans blue dye25. LPS administration significantly increased vascular permeability in the kidney compared with control. Pre-treatment or post-treatment with resveratrol significantly decreased the LPS-induced renal vascular permeability (Fig. 3C).
Resveratrol treatment lowers renal cytokine concentrations in mice exposed to LPS
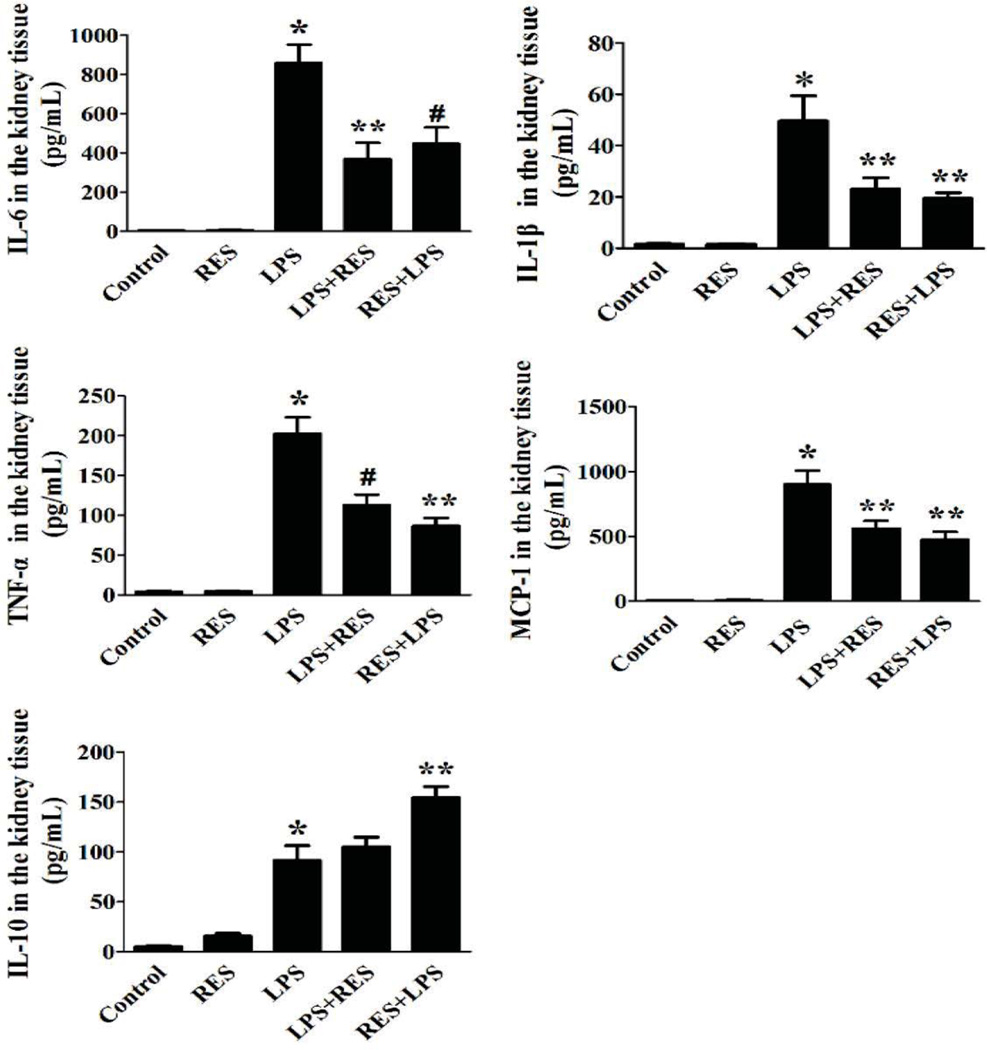
We further evaluated the anti-inflammatory effect of resveratrol on LPS induced AKI by measuring the concentration of several cytokines in kidney tissue. The result from ELISA demonstrated that the pro-inflammatory cytokines markedly increased in kidney with administration of LPS, however resveratrol treatment resulted in suppression of cytokines within the kidney similar to that of the serum cytokines. IL-10 in kidney was also measured to evaluate the potential anti-inflammatory response. IL-10 greatly increased in the pre-treatment group and slightly increased in the post-treatment group compared with the untreated LPS group. In mice not exposed to LPS, resveratrol alone did not impact the IL-10 concentration compared with the control group (Fig. 4).
Figure 4. Resveratrol lowers renal cytokine concentrations and reduces infiltrating inflammatory cell populations in the kidney.
A. The concentration of IL-1β, IL-6, MCP-1, TNFα and IL-10 in kidney tissue from each group was measured by ELISA. The groups were designated as follows, LPS: LPS injected group; RES: Resveratrol only group; RES + LPS: LPS + Resveratrol pre-treatment group; LPS + RES: LPS + Resveratrol post-treatment group. Data were presented as mean ± SEM. (n=8, *p<0.01 vs. Control, **p<0.01 vs. LPS, #p<0.05 vs. LPS).
Resveratrol inhibits macrophage activation and induces apoptosis of macrophages
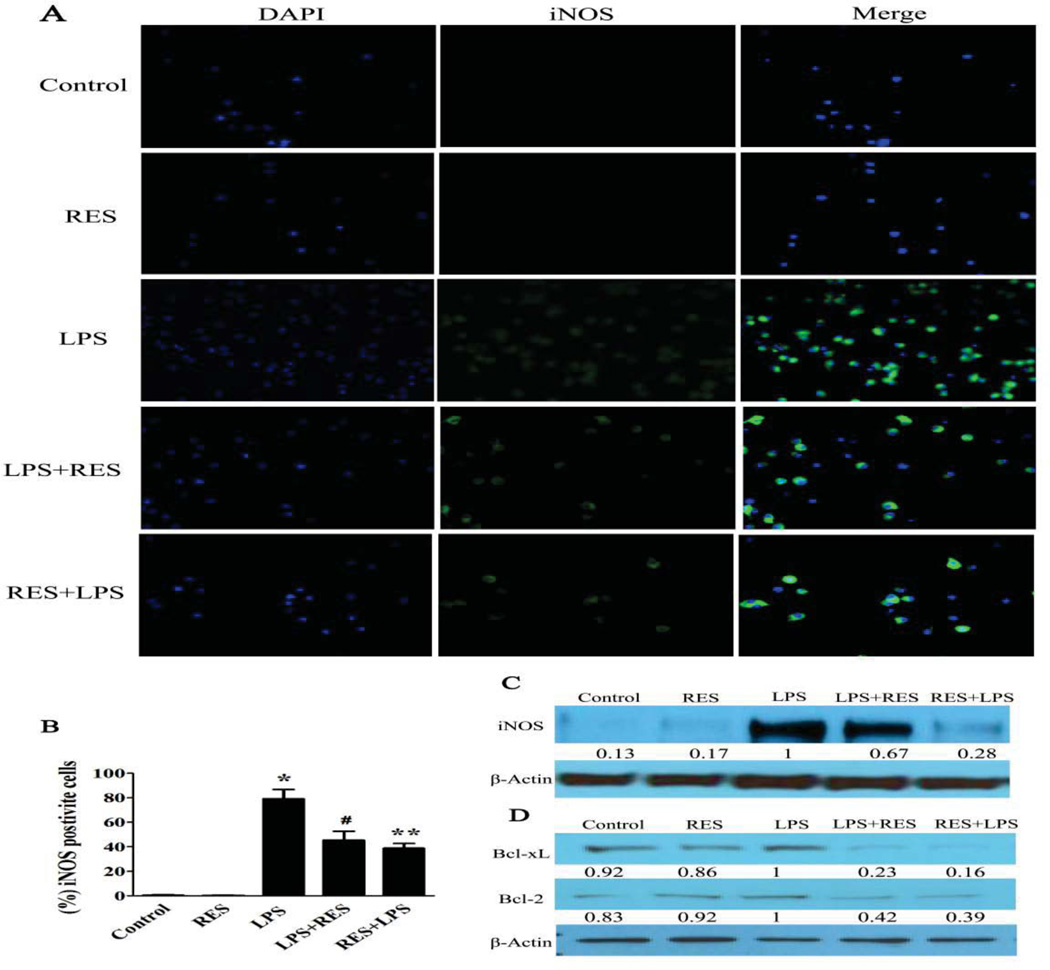
To investigate whether decreasing infiltrating inflammatory cells is associated with decreases in macrophages activity, we assessed the polarization of macrophages in each treatment group using immunohistochemical analysis. The iNOS-positive macrophages, which represent activated macrophages, markedly increased in the LPS group. However, the percentage of activated macrophages significantly decreased in the resveratrol pre-treatment and post-treatment groups (Fig. 5A). The expression of iNOS in macrophages was assessed by western blot. LPS exposure resulted in a dramatic increase in iNOS. The concentration of iNOS was reduced slightly by resveratrol post-treatment and more markedly with pre-treatment (Fig. 5A–C). The status of renal macrophages was also evaluated by western blot, with Bcl-2 and Bcl-xL increasing in the untreated LPS group and resveratrol treatment ameliorating this effect in both pre-treatment and post-treatment groups (Fig. 5D). Of note, resveratrol alone showed little effect on the concentration of apoptosis-related proteins compared with the control group. These results suggested that resveratrol not only inhibits macrophage activation but also induces apoptosis in activated macrophages.
Figure 5. Resveratrol inhibits macrophages activation and induces macrophage apoptosis.
iNOS, a marker of macrophages activation, was evaluated in macrophages. A. The expression level of iNOS (green) in macrophages isolated from different groups was assessed by immunofluorescence. Representative immunofluorescence images were taken at 400×. DAPI (blue) was used as a nuclear counterstain. B. A graph of the percentage of iNOS positive macrophages in at least 100 counted cells. C. Western blot to evaluate the expression level of iNOS in cell lysates using relevant antibodies. D. Western blot to evaluate the expression level of Bcl-2 and Bcl-xL in cell lysates using relevant antibodies. β-actin was used as a standard control. The fold change was compared with the LPS group (as 1.0). The groups were designated as follows, LPS: LPS injected group; RES: Resveratrol only group; RES + LPS: LPS + Resveratrol pre-treatment group; LPS + RES: LPS + Resveratrol post-treatment group. Data were presented as mean ± SEM. (n=8, *p<0.01 vs. Control, **p<0.01 vs. LPS, #p<0.05 vs. LPS).
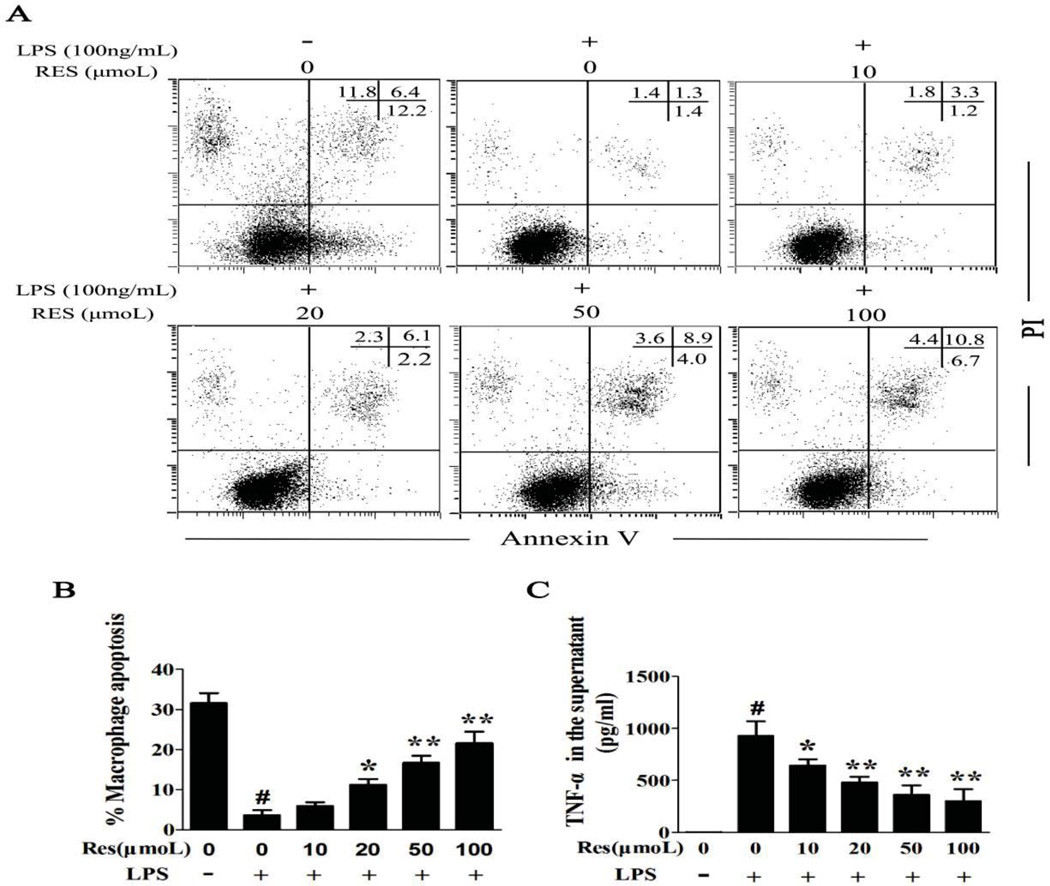
Resveratrol induces apoptosis and lowers TNFα release in LPS activated BMDMs
In order to gain a better understanding of the mechanism immunomodulatory effect of resveratrol on macrophages, an in vitro assay was employed. BMDMs were stimulated with (100 ng/ml) LPS for 2 h, then incubated with various concentrations of resveratrol for 24 h. BMDMs were evaluated by Annexin V and PI dual staining to quantify apoptosis (Fig. 6A). As is seen in (Fig. 6B), BMDMs showed spontaneous apoptosis in culture which was rescued following activation with LPS stimulation suggesting that LPS prolongs the survival time of BMDM. In contrast, resveratrol treatment increased the percentage of BMDM apoptosis compared with the untreated LPS group in a concentration dependent fashion. It is well known that in addition to activating macrophages, cytokines also prolong macrophage function and infer resistance against apoptosis during an immune response [26, 27]. Hence, the concentration of TNFα in the supernatant, which is essential for long-term survival of macrophages, was measured [28]. The result from ELISAs showed that TNFα increases in the LPS group and it decreased with increasing concentration of resveratrol (Fig. 6C). These data suggested that resveratrol modulates the immune response in AKI by suppressing the macrophage response to LPS.
Figure 6. Resveratrol induces apoptosis in activated BMDM and lowers TNFα release of BMDM induced by LPS.
Using an in vitro assay, BMDMs were pre-incubated with LPS (100 ng/ml) for 2 h, then various concentrations of resveratrol (10, 20, 50, 100 µmol) were added. After an additional 24 h, the medium and cells were collected for follow-up assay. A. Apoptosis was evaluated by flow cytometry. B. The percentage of apoptotic cells was determined by Annexin V/PI staining. The cells that were positive for Annexin V, PI, or both were considered apoptotic. C. The concentration of TNFα in the medium was measured by ELISA. Data were presented as mean ± SEM. (n=6, in triplicate; #p<0.05 vs. Control; *p<0.05 or **p<0.01 vs. LPS only).
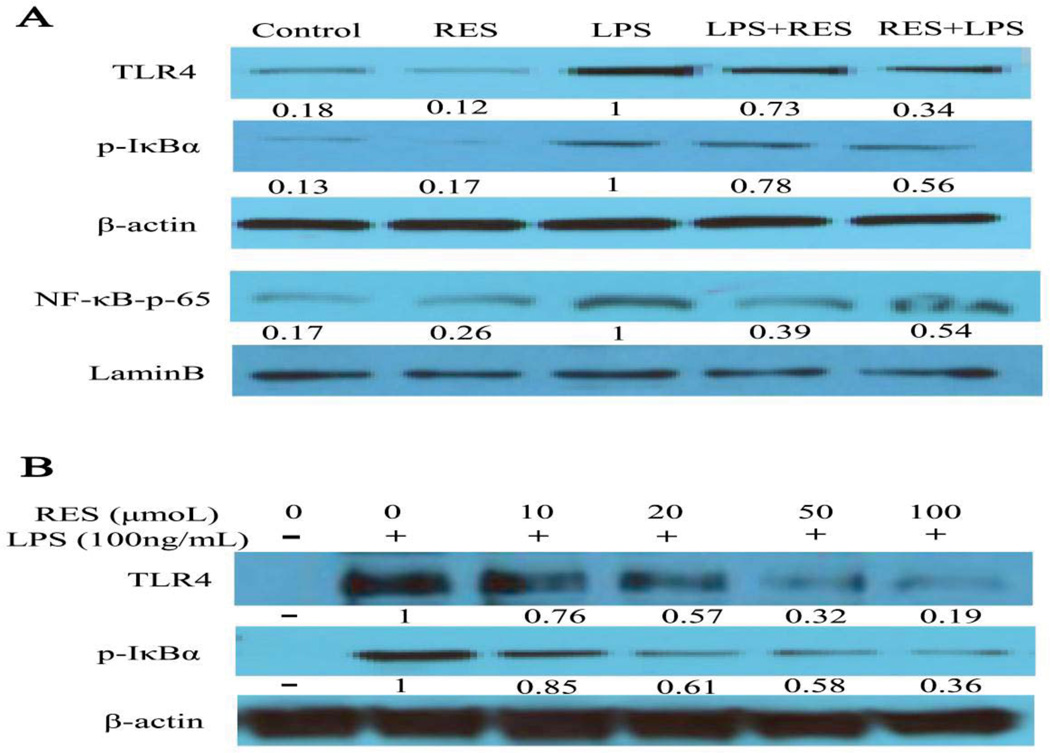
Resveratrol modulates LPS-stimulated macrophages by TLR4-NF-B signalling
TLR4-NF-κB plays a critical role in the pro-inflammation cytokine production of macrophages induced by LPS [22, 23]. Therefore, we assessed the expression of TLR4 and NF-κB in vivo and in vitro. Our data showed that the expression of TLR4 protein was significantly inhibited by resveratrol, especially in the pre-treatment group. We also assessed the phosphorylation of the inhibitor of NF-κB (IκBα) and the nuclear translocation of NF-κB p65 for NF-κB activity. We found the expression of p-IκBα was increased in the LPS group, whereas it was decreased in both resveratrol treatment groups. Similarly, the expression of NF-κB p65 in nuclear protein, increased in the LPS group and decreased in resveratrol pre-treatment and post-treatment groups (Fig. 7A). We also found that resveratrol significantly suppressed TLR4 expression in a concentration-dependent manner in vitro and down-regulated p-IκBα after LPS exposure (Fig. 7B), similar to the observations in vivo. These data suggested that resveratrol modulated macrophage responses to LPS through the TLR4-NF-κB signaling pathway
Figure 7. Resveratrol modulates LPS-stimulated macrophages by TLR4-NF-κB signaling.
A. Using an in vivo assay, mice were treated as above, and the macrophages isolated from the kidneys of mice from each group. The protein was prepared for western blot to evaluate the expression levels of TLR4 and p-IκBα in cell lysates using relevant antibodies. β-actin was used as a standard control. NF-κB-p65 was used to evaluate the activity of NF-κB in nuclear extracts and Lamin-B was used as a standard control. The groups were designated as follows, LPS: LPS injected group; RES: Resveratrol only group; RES + LPS: LPS + Resveratrol pre-treatment group; LPS + RES: LPS + Resveratrol post-treatment group. B. In vitro assay, BMDM were treated as described in Figure 6, the expression level of TLR4 and p-IκBα in cell lysates using relevant antibodies, β-actin was used as a standard control. The fold change was compared with the LPS group in vivo or the LPS only group in vitro (as 1.0).
Discussion
AKI, the devastating clinical syndrome characterized by a rapid renal failure, is a leading cause of the morbidity and mortality of patients with severe sepsis [2]. Hypoperfusion caused by global or regional hemodynamic dysfunction is generally considered a critical pathophysiology for septic AKI. However, early goal-directed therapy has shown little improvement in the mortality rates of AKI [29, 30]. One explanation for this is that renal damage might occur before hemodynamic alteration. This point is supported by a previous study showing that within 10 minutes after intravenous LPS injection, endotoxin was visualized in the renal interstitium [31]. Thus, the goal of the current study was not only to investigate resveratrol as a potential therapeutic approach for LPS induced AKI, but also to uncover the mechanism of sepsis induced AKI. Our study, to the author's knowledge, demonstrates for the first time that resveratrol acts to modulate the immune response to protect the kidneys from LPS induced injury by multiple pathways, including lowering cytokine concentrations, reducing vascular permeability, suppressing macrophage activation and inducing macrophage apoptosis.
Numerous studies in animal models have demonstrated that resveratrol exhibits beneficial effects on renal function [32, 33]. In these experiments, resveratrol was employed as a post-treatment, after the induction of disease. -However, induction of cytokines, a hallmark of sepsis, triggers a secondary inflammatory cascade, which results in subsequent immune dysfunction. Hence, in our study, we administered resveratrol as both a pre-LPS treatment and a post-LPS treatment. The pre-treatment group had significantly lower serum cytokine concentrations and greater renal protection compared with the post-treatment group, which may be attributed to the inhibition by resveratrol of early cytokine production and secondary inflammatory signal amplification. These results are consistent with other research suggesting that an abnormal immune response might be a major factor associated with the early development of AKI [34, 35].
Endothelial cell permeability plays an important role in facilitating early inflammatory responses in the kidney. Increasing the permeability of the endothelial monolayer aids immune cell migration into the inflamed sites to fight infection and promote the tissue repair process [36]. However, LPS significantly increased vascular permeability and excessive inflammatory cell infiltration [37]. This leads to local microcirculatory hypoperfusion, hypoxia and mitochondrial dysfunction, all of which contribute to the progression of AKI [38]. In our study, we found that resveratrol treatment significantly inhibited renal vascular endothelial cell permeability and infiltration of inflammatory cells, such as granulocytes and macrophages, into the renal interstitium. This is associated with decreased levels of cytokines in renal tissue, therefore markedly alleviating the local immune response induced by LPS.
Macrophages are present in healthy kidney tissue, and ultimately carry out the initiation, propagation, and resolution phases of AKI. During renal inflammation, macrophages are divided into groups dependent on phenotype and function: classically activated (M1) and alternatively activated (M2) [10, 11, 13]. Similar to a previous study, we found that macrophages in LPS induced AKI acquired an M1 phenotype represented as increased iNOS expression, dominating the early inflammatory stage [39]. To our surprise, resveratrol administration significantly suppressed macrophage activation, and induced apoptosis in activated macrophages coupled with decreased concentrations of Bcl-xL and Bcl-2. It is well known that TNFα not only directs induction of apoptosis in renal vascular endothelial cells and tubular epithelial cells, but is also central to macrophage survival and function [26, 28, 40, 41]. In our study, we found that resveratrol induced apoptosis in activated macrophages in a concentration fashion in vitro, correlated with reduced TNFα concentration. Thus, we speculate that, before LPS injection, the status of macrophages is similar because resveratrol alone does not induce macrophage apoptosis in vivo. However, after LPS treatment, resveratrol suppressed TNFα release by macrophages and he decreased level of TNFα in turn resulted in reduced function and lifetime of macrophages. Therefore, resveratrol could be directly involved in establishing a feedback loop that reduces further recruitment of inflammatory cells into the kidneys. This is supported by other studies showing that M1 macrophages mainly determine the outcome of AKI [39, 42]. At the same time, we also found the anti-inflammatory cytokine IL-10 significantly increased in the kidney upon resveratrol treatment, which is associated with an anti-inflammatory effect of M2 macrophages. M2 macrophages play a key role in renal repair after inflammation and promote the progression of renal fibrosis [10, 43, 44]. Further studies in this area should be designed to investigate whether resveratrol can modulate the M1–M2 phenotype switch and effectively block TGF-β, which is released by M2 macrophages and is involved in the progression of kidney fibrosis in AKI.
TLR4 is a cellular receptor for LPS. Two downstream pathways, MyD88- and TRIF-signaling, are involved with TLR4 activation by LPS, and culminate in NF-κB expression [14, 27]. Several studies have demonstrated that resveratrol exerts its anti-inflammatory effect on RAW 264.7 macrophages by TLR4-NF-κB pathway [22, 23]. In the current study, we found that resveratrol inhibited TLR4 and NF-κB activity in kidney macrophages. Moreover, we also found that resveratrol suppresses the expression level of TLR4 and p-IκBα in macrophages in vitro. Taken all together, these results suggest that resveratrol regulates LPS-induced macrophage activation and survival by the TLR4-NF-κB pathway.
In summary, our results demonstrated that resveratrol is effective in reducing the renal immune dysfunction induced by LPS, thus protecting the kidney from injury due to excessive inflammation. Moreover, resveratrol acts on multiple pathways that resulted in the alleviation of LPS-induced inflammatory response in AKI, including (i) suppression of pro-inflammatory macrophages, (ii) induction of apoptosis in activated macrophages, and (iii) suppression of endothelial cell dysfunction. Resveratrol may therefore be a novel anti-inflammatory therapy to prevent LPS-induced AKI.
Acknowledgments
LC, SY, PSN and MN conceived and designed the experiments. LC performed the experiments. LC, SY, HG, PSN and MN gave technical support and conceptual advice. LC, HG, PSN and MN contributed reagents, tools and materials. Interpreted the data and wrote the paper: LC, SY, EEZ, PSN and MN.
This work was supported in part by National Institutes of Health grants P01AT003961, R01AT006888, R01ES019313, R01MH094755, and P20GM103641 as well as by Veterans Affairs Merit Award BX001357.
Abbreviations
- AKI
acute kidney injury
- BUN
blood urea nitrogen
- BMDM
bone marrow-derived macrophages
- FBS
fetal bovine serum
- IκBα
inhibitor of NF-κB
- MCP-1
monocyte chemotactic protein
- M-CSF
murine macrophage colony-stimulating factor
- NF-κB
nuclear factor-κB
- ROS
reactive oxygen species
- RBF
renal blood flow
- TLR-4
toll-like receptor 4
- (TNF-a)
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/mnfr.201400819.
The authors declare no conflict of interest.
References
- 1.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 2.Zarjou A, Agarwal A. Sepsis and acute kidney injury. Journal of the American Society of Nephrology : JASN. 2011;22:999–1006. doi: 10.1681/ASN.2010050484. [DOI] [PubMed] [Google Scholar]
- 3.Rivers EP, Coba V, Whitmill M. Early goal-directed therapy in severe sepsis and septic shock: a contemporary review of the literature. Current opinion in anaesthesiology. 2008;21:128–140. doi: 10.1097/ACO.0b013e3282f4db7a. [DOI] [PubMed] [Google Scholar]
- 4.Otero RM, Nguyen HB, Huang DT, Gaieski DF, et al. Early goal-directed therapy in severe sepsis and septic shock revisited: concepts, controversies, and contemporary findings. Chest. 2006;130:1579–1595. doi: 10.1378/chest.130.5.1579. [DOI] [PubMed] [Google Scholar]
- 5.Langenberg C, Wan L, Egi M, May CN, Bellomo R. Renal blood flow in experimental septic acute renal failure. Kidney international. 2006;69:1996–2002. doi: 10.1038/sj.ki.5000440. [DOI] [PubMed] [Google Scholar]
- 6.Wan L, Bagshaw SM, Langenberg C, Saotome T, et al. Pathophysiology of septic acute kidney injury: what do we really know? Critical care medicine. 2008;36:S198–S203. doi: 10.1097/CCM.0b013e318168ccd5. [DOI] [PubMed] [Google Scholar]
- 7.Chvojka J, Sykora R, Karvunidis T, Radej J, et al. New developments in septic acute kidney injury. Physiological research / Academia Scientiarum Bohemoslovaca. 2010;59:859–869. doi: 10.33549/physiolres.931936. [DOI] [PubMed] [Google Scholar]
- 8.Legrand M, Bezemer R, Kandil A, Demirci C, et al. The role of renal hypoperfusion in development of renal microcirculatory dysfunction in endotoxemic rats. Intensive care medicine. 2011;37:1534–1542. doi: 10.1007/s00134-011-2267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. The Journal of clinical investigation. 2009;119:2868–2878. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lech M, Anders HJ. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochimica et biophysica acta. 2013;1832:989–997. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Nelson PJ, Rees AJ, Griffin MD, Hughes J, et al. The renal mononuclear phagocytic system. Journal of the American Society of Nephrology : JASN. 2012;23:194–203. doi: 10.1681/ASN.2011070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin MD. Mononuclear phagocyte depletion strategies in models of acute kidney disease: what are they trying to tell us? Kidney international. 2012;82:835–837. doi: 10.1038/ki.2012.164. [DOI] [PubMed] [Google Scholar]
- 13.Alagesan S, Griffin MD. Alternatively activated macrophages as therapeutic agents for kidney disease: in vivo stability is a key factor. Kidney international. 2014;85:730–733. doi: 10.1038/ki.2013.405. [DOI] [PubMed] [Google Scholar]
- 14.El-Achkar TM, Hosein M, Dagher PC. Pathways of renal injury in systemic gram-negative sepsis. European journal of clinical investigation. 2008;38(Suppl 2):39–44. doi: 10.1111/j.1365-2362.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- 15.Verstrepen L, Bekaert T, Chau TL, Tavernier J, et al. TLR-4, IL-1R and TNF-R signaling to NF-kappaB: variations on a common theme. Cellular and molecular life sciences : CMLS. 2008;65:2964–2978. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayeux PR, MacMillan-Crow LA. Pharmacological targets in the renal peritubular microenvironment: implications for therapy for sepsis-induced acute kidney injury. Pharmacology & therapeutics. 2012;134:139–155. doi: 10.1016/j.pharmthera.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong S, Lu Y. Omega-3 fatty acid-derived resolvins and protectins in inflammation resolution and leukocyte functions: targeting novel lipid mediator pathways in mitigation of acute kidney injury. Frontiers in immunology. 2013;4:13. doi: 10.3389/fimmu.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nature reviews. Drug discovery. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 19.Rieder SA, Nagarkatti P, Nagarkatti M. Multiple anti-inflammatory pathways triggered by resveratrol lead to amelioration of staphylococcal enterotoxin B-induced lung injury. British journal of pharmacology. 2012;167:1244–1258. doi: 10.1111/j.1476-5381.2012.02063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holthoff JH, Wang Z, Seely KA, Gokden N, Mayeux PR. Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney international. 2012;81:370–378. doi: 10.1038/ki.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitada M, Koya D. Renal protective effects of resveratrol. Oxidative medicine and cellular longevity. 2013;2013:568093. doi: 10.1155/2013/568093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakus PB, Kalman N, Antus C, Radnai B, et al. TRAF6 is functional in inhibition of TLR4-mediated NF-kappaB activation by resveratrol. The Journal of nutritional biochemistry. 2013;24:819–823. doi: 10.1016/j.jnutbio.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Li S, Yang Q, Shi Y, et al. Resveratrol reduces the proinflammatory effects and lipopolysaccharide- induced expression of HMGB1 and TLR4 in RAW264.7 cells. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2014;33:1283–1292. doi: 10.1159/000358696. [DOI] [PubMed] [Google Scholar]
- 24.Mustafa A, McKallip RJ, Fisher M, Duncan R, et al. Regulation of interleukin-2-induced vascular leak syndrome by targeting CD44 using hyaluronic acid and anti-CD44 antibodies. Journal of immunotherapy. 2002;25:476–488. doi: 10.1097/00002371-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators of inflammation. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flad HD, Grage-Griebenow E, Petersen F, Scheuerer B, et al. The role of cytokines in monocyte apoptosis. Pathobiology : journal of immunopathology, molecular and cellular biology. 1999;67:291–293. doi: 10.1159/000028082. [DOI] [PubMed] [Google Scholar]
- 27.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Lombardo E, Alvarez-Barrientos A, Maroto B, Bosca L, Knaus UG. TLR4-mediated survival of macrophages is MyD88 dependent and requires TNF-alpha autocrine signalling. Journal of immunology. 2007;178:3731–3739. doi: 10.4049/jimmunol.178.6.3731. [DOI] [PubMed] [Google Scholar]
- 29.Dudley C. Maximizing renal preservation in acute renal failure. BJU international. 2004;94:1202–1206. doi: 10.1046/j.1464-410x.2004.05129.x. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen HB, Corbett SW, Menes K, Cho T, et al. Early goal-directed therapy, corticosteroid, and recombinant human activated protein C for the treatment of severe sepsis and septic shock in the emergency department. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2006;13:109–113. doi: 10.1197/j.aem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 31.El-Achkar TM, Huang X, Plotkin Z, Sandoval RM, et al. Sepsis induces changes in the expression and distribution of Toll-like receptor 4 in the rat kidney. American journal of physiology. Renal physiology. 2006;290:F1034–F1043. doi: 10.1152/ajprenal.00414.2005. [DOI] [PubMed] [Google Scholar]
- 32.Palsamy P, Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochimica et biophysica acta. 2011;1812:719–731. doi: 10.1016/j.bbadis.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Shi YW, Wang CP, Liu L, Liu YL, et al. Antihyperuricemic and nephroprotective effects of resveratrol and its analogues in hyperuricemic mice. Molecular nutrition & food research. 2012;56:1433–1444. doi: 10.1002/mnfr.201100828. [DOI] [PubMed] [Google Scholar]
- 34.Benes J, Chvojka J, Sykora R, Radej J, et al. Searching for mechanisms that matter in early septic acute kidney injury: an experimental study. Critical care. 2011;15:R256. doi: 10.1186/cc10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holly MK, Dear JW, Hu X, Schechter AN, et al. Biomarker and drug-target discovery using proteomics in a new rat model of sepsis-induced acute renal failure. Kidney international. 2006;70:496–506. doi: 10.1038/sj.ki.5001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutton TA, Dagher PC. Fueling the fire in acute kidney injury: endothelial cells collect their Toll. Kidney international. 2011;79:267–269. doi: 10.1038/ki.2010.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim DH, Jung YJ, Lee AS, Lee S, et al. COMP-angiopoietin-1 decreases lipopolysaccharide-induced acute kidney injury. Kidney international. 2009;76:1180–1191. doi: 10.1038/ki.2009.387. [DOI] [PubMed] [Google Scholar]
- 38.Ergin B, Kapucu A, Demirci-Tansel C, Ince C. The renal microcirculation in sepsis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014 doi: 10.1093/ndt/gfu105. [DOI] [PubMed] [Google Scholar]
- 39.Lee S, Huen S, Nishio H, Nishio S, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. Journal of the American Society of Nephrology : JASN. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu C, Chang A, Hack BK, Eadon MT, et al. TNF-mediated damage to glomerular endothelium is an important determinant of acute kidney injury in sepsis. Kidney international. 2014;85:72–81. doi: 10.1038/ki.2013.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Misseri R, Meldrum DR, Dinarello CA, Dagher P, et al. TNF-alpha mediates obstruction-induced renal tubular cell apoptosis and proapoptotic signaling. American journal of physiology. Renal physiology. 2005;288:F406–F411. doi: 10.1152/ajprenal.00099.2004. [DOI] [PubMed] [Google Scholar]
- 42.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney international. 2011;80:915–925. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- 43.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. The Journal of clinical investigation. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duffield JS. Macrophages and immunologic inflammation of the kidney. Seminars in nephrology. 2010;30:234–254. doi: 10.1016/j.semnephrol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]