Abstract
Background
Premenopausal migraines frequently are associated with fluctuations of estrogen levels. Both, migraine and combined hormonal contraceptives (CHC) increase the risk of vascular events. Therefore progestagen-only contraceptives (POC) are a safer alternative. A previous short-term study demonstrated a positive impact of the oral POC desogestrel on migraine frequency. To study the effect of the POC desogestrel 75 μg on migraine frequency, intensity, use of acute medication and quality of life in a clinical setting over the period of 180 days.
Methods
Patients’ charts were screened for women with migraine, who had decided to use desogestrel for contraception. Charts were included, if routinely conducted headache diaries were complete for 90 days before treatment (baseline) and over a treatment period of 180 days. We also report about starters who stopped treatment early, because of adverse events. Baseline data (day 1–90 before treatment) were compared with first and second treatment period (treatment days 1–90 and days 91–180). Quality of life was evaluated using MIDAS questionnaires.
Results
Days with migraine (5.8 vs 3.6), with any kind of headache (9.4 vs 6.6), headache intensity (15.7 vs 10.7), days with severe headache (5.4 vs 2.4) and use of triptans (12.3 vs7.8) were significantly reduced after 180 days. MIDAS score and grade improved significantly.
Conclusion
Contraception with desogestrel 75 μg resulted in a significantly improved quality of life and a reduction of migraine days over the observation period of 180 days. A clinically meaningful 30% reduction in pain was observed in 25/42 (60%) participants. For counselling reasons it is of importance, that the major reduction in migraine frequency occured during the initial 90 days, however further improvement occurs with longer duration of use. Prospective studies are needed to confirm these results.
Keywords: Hormonal migraine, Contraception, Progestagen-only pill, Desogestrel, Migraine without aura, Headache, Migraine with aura, Cardiovascular risk, Triptans
Background
Epidemiological data suggest that combined hormonal contraceptives (CHC) initiate or worsen migraine and headache in predisposed women [1-5]. The incidence of migraine is highest during the reproductive years and more than 50% of women report an association between migraine attacks and their menstrual cycle [6,7]. The reproductive phase is also the life span in which most women need efficient contraception. Migraine with aura (MA) and to a lesser extent migraine without aura (MO) increase the risk for cardiovascular events, especially for stroke [8-11]. There is a substantial elevation of these risks in migraineurs using CHC [11-14]. The cardiovascular risk associated with CHC, has been mainly attributed to the estrogen component which exerts a strong effect on the coagulation system. Finding a well-tolerated estrogen-free form of contraception for headache patients therefore is an important issue.
Progestagen-only pills (POP) have so far not been found to be associated with an increased risk for thromboembolic or ischemic events [15]. Most guidelines recommend progestagen-only contraception as a safer option [16]. The POP desogestrel 75 μg (Cerazette®; MSD Merck Sharp & Dohme AG, Luzern, Switzerland) is used continuously and combines efficient inhibition of ovulation with maintenance of low estrogen levels [17,18]. Avoidance of estrogen peaks and withdrawal could contribute to good tolerability of this contraceptive in migraineurs. Recently we reported a benefit of desogestrel 75 μg on migraine and quality of life over a 3 month period of use [19,20]. The effect on frequency and quality of life was comparable to improvements observed with prophylactic agents. However, the observation interval was short. In the present study, we report effects of 6 cycles desogestrel contraception on headache frequency, intensity and use of pain medication.
Methods
This study was performed at the divison for family planning, unit of the Department of Reproductive Endocrinology, University Hospital Zürich, Switzerland where one of the authors (GM) runs an outpatient clinic for migraine patients with need for hormonal therapy. Migraine is diagnosed according to the IHS (International Headache Society) criteria by the referring neurologists from headache centres in Zürich, Bad Zurzach or by the author [21]. Reasons for referral were need for contraception in women with migraine, menstrual migraine or any form of hormonal therapy of headaches. To allow an exact diagnosis of the headache type and frequency according to the IHS our patients are principally instructed to conduct headache diaries for 3 cycles before their first visit and to continue after any intervention. MIDAS questionnaires are used before interventions and in intervals of 90 days thereafter. The majority of our premenopausal patients have a need for efficient contraception. In the context of the discussions around the elevated risks for cardiovascular disease and stroke we advise against combined hormonal contraceptives as a first choice contraception in migraineurs and in women aged 35 years or more. Before starting a hormonal treatment women are informed about risks and potential side effects which include information about irregular bleeding and acne with the use of desogestrel.
For the present study patients’ charts were screened for women with migraine, who had decided to use the POP desogestrel 75 μg and had conducted headache diaries 90 days before initiation and over 180 days of use of this medication. We included patients suffering from all types of migraine. The observation period was defined from July 2009 to December 2013. In a previous study we already reported 90 day treatment data of 16 included patients. Women had to be premenopausal and had to need effective contraception. We report about all adverse events causing discontinuation earlier than 180 days. Exclusion criteria were: incomplete diaries, less than 10 headache episodes during the pretreatment period, initiation or change of prophylactic medications during the observation and postmenopause. This resulted in a drop-out rate of 26 out of 68 charts.
The diaries include information on the number of migraine and headache days, the severity of headache, the use of triptans and other pain medication, the use of hormones and days with vaginal bleeding. Days of bleeding were assessed to allow an exact diagnosis of the migraine type according to the IHS criteria. Headache severity was rated in the diaries according to a 4-point scale (0 = no pain, 3 = severe pain). This score is easy to understand and has been proven to be useful in daily work with migraineurs. For ethical reasons all diaries were anonymised before data evaluation. The evaluation of anonymised data in our setting was accepted by the ethical committee of the Kanton Zürich.
Primary efficacy variables were the differences in number of migraine and headache days, the difference in pain score as well as MIDAS score and grade. Secondary outcomes included differences in the number of all pain medications and triptans used as well as differences in days with pain score three. In population-based studies of migraine- and headache sufferers in the US and UK the MIDAS questionnaire and the MIDAS summary scores proved to be a highly reliable means of assessment of the impact of the ailment on daily life [22,23]. The total MIDAS score strongly correlates with both the clinical evaluation of the severity of a patient’s headache problem, and the frequency of the episodes, determined from daily-based headache diaries [23].
Statistical analyses
Data were compared between baseline (BL) (day 1–90 before treatment) and treatment periods (TP): TP1 (day 1–90) TP2 (day 91–180). In addition all variables were compared between TP1 and TP2. Statistical analyses were done using IBM SPSS Statistics, version 22 (Armonk, New York, IBM Corp). Data are presented as mean (SD). Pain intensity score was calculated as the sum of headache intensities for baseline and each treatment period according to the above mentioned 4-point scale. For each period this sum was divided by three to obtain a mean monthly pain score. To calculate monthly frequencies, the numbers for each observational episode was divided by three. Numbers of monthly migraine days, headache days, headache intensity, days with use of pain medication and questions of the MIDAS questionnaire were compared with Friedman’s test. Post-hoc comparisons between single time points were performed using Wilcoxon’s signed rank test with Bonferroni correction.
Results
A total of 68 women with migraine initiated contraception with desogestrel 75 μg. Headache diaries of 42 subjects were complete and eligible for analysis. Six patients had stopped desogestrel because of side effects within 42 or less days and were excluded (prolonged bleeding n = 3, increase of headache n = 2, acne = 1) (Figure 1). Demographics and characteristics of eligible women and drop-outs did not differ significantly (Table 1). Hormonal contraception was used by 50% (n = 21) of the included patients and 61% (n = 16) in the drop-out (p > 0.05). One included woman had used a copper-device (drop-outs: n = 0). Chronic headaches (>15 /month) were found in 6 included patients and more than 8 triptans were used monthly by 9 included patients. Mean age of migraine onset was 22.4 years (SD 5.2). Two women suffered from endometriosis. Frequency of migraine, headache intensity, days with use of pain medication and triptans were significantly reduced during TP2 in comparison with BL (Table 2). Days with severe pain declined from 5.4 (SD 4.2) to 2.4 (SD 3.5) (p < 0.001) (Table 2). The improvements were in large parts visible during TP1 and persisted during further follow-up. A according to the IHS clinically meaningful 30% reduction in pain was observed in 25/42 (60%) participants, whereas another 28% (12/42) experienced even a 50% reduction [24,25]. We found a 255 reduction in the sum of headache and migraine days in 55% (23/42) of the included migraineurs. Seven of 42 patients (16%) experienced 1–5 more headache/migraine days during TP2 in comparison to BL. Interestingly, however, quality of life improved in five of these seven women. Further analyses to explain this seemingly contradictory result revealed a decrease in days with pain score 3 and a decrease in overall pain intensity in all these five patients. Two women with more migraine attacks and without improvement in the MIDAS score, decided to change to a non-hormonal contraception after 180 days.
Figure 1.
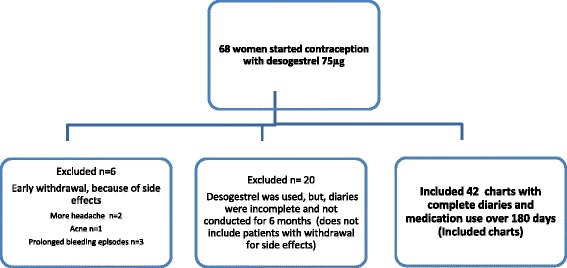
Flow diagram of the study population.
Table 1.
Demographic and baseline characteristics of included charts (n = 42) and excluded charts (n = 26)
| Demographics | Included patients n = 42 | Drop-out group n = 26 | P - value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (years) | 35.1 (8.9) | 31.1 (9.8) | 0.64 |
| Height (cm) | 165.7 (6.5) | 166.2 (5.7) | 0.78 |
| Weight (kg) | 60.6 (8.4) | 61.6 (9.0) | 0.37 |
| Systolic blood pressure (mmHG) | 119.9 (12.6) | 114.7 (26.1) | 0.19 |
| Diastolic blood pressure (mmHG) | 73.7 (9.9) | 75.7 (9.6) | 0.91 |
| Baseline characteristics | |||
| Migraine days per month | 5.8 (4.3) | 6.6 (3.6) | 0.62 |
| Headache Intensity /month | 15.7 (7.6) | 20.3 (9.5) | 0.28 |
| Age of migraine onset | 22.4 (5.2) | 24.2 (7.2) | 0.42 |
| Triptan users | 12.3 (15.0) | 12.1 (11.7) | 0.71 |
| MIDAS: Headache days | 25.2 (17.2) | 23.0 (12.8) | 0.37 |
| MIDAS: (Pain intensity) | 6.4 (1.7) | 6.7 (1.6) | 0.57 |
| MIDAS: Grade | 3.5 (0.7) | 3.3 (0.8) | 0.38 |
| Number (%) | Number (%) | ||
| Migraine with aura | 10 (24) | 10 (38) | 0.19 |
| Migraine without aura | 32 (76) | 16 (61.1) |
Table 2.
Changes in migraine, frequency, intensity and use of pain medication during use of the contraceptive pill desogestrel 75 μg over 180 days of use
| Days/month | Mean baseline (SD) | Mean treatment TP 1(SD) | Mean treatment TP 2 (SD) | Overall P-value | P-value baseline vs. TP 1 | Posthoc* P-value baseline vs. TP2 | P-value TP 1 vs TP 2 |
|---|---|---|---|---|---|---|---|
| Headache days | 4.1 (4.5) | 3.8 (4.1) | 3.0 (4.1) | 0.051 | 0.867 | 0.050 | 0.062 |
| Migraine days | 5.8 (4.3) | 3.7 (3.4) | 3.6 (4.0) | <0.001 | <0.001 | 0.001 | 0.701 |
| Sum headache and migraine | 9.4 (5.1) | 7.6 (5.5) | 6.6 (5.4) | 0.002 | 0.008 | <0.001 | 0.130 |
| Headache intensity | 15.7 (7.6) | 11.4 (7.5) | 10.7 (8.0) | <0.001 | <0.001 | <0.001 | 0.312 |
| Days with headache score 3 in 3 months | 5.4 (4.2) | 2.2 (2.7) | 2.4 (3.5) | <0.001 | <0.001 | <0.001 | 0.776 |
| Pain medication | 7.2 (5.9) | 5.0 (3.3) | 5.0 (4.0) | 0.044 | <0.001 | 0.015 | 0.751 |
| Triptan use in 3 months | 12.3 (15.0) | 8.1 (9.4) | 7.8 (10.2) | 0.035 | 0.010 | 0.041 | 0.599 |
Baseline: 90 days before treatment; TP = treatment period. TP1: Treatment period days 1–90; TP2: Treatment period days 91–180.
*after Bonferroni correction, post-hoc p-values are significant at p < 0.017.
Table 3 demonstrates the changes in quality of life. All MIDAS items improved significantly during 180 days of desogestrel use (TP2). Again significant improvement was already observed after TP1. Separate analyses for MO and MA women revealed no differences with regard to demographic parameters between the groups. In MO patients significant improvements of all features (except headache days) days were observed (Table 4). The very small group of subjects with MA experienced significant reductions in the number of pain medications and triptans, MIDAS score and MIDAS grade.
Table 3.
Changes in quality of life measured with the MIDAS after 90 days and 180 days contraception with desogestrel 75 μg
| N = 42 | Mean (SD) Baseline | Mean (SD) TP 1 | Mean (SD) TP 2 | Overall p-value | P-value baseline vs. TP1 | Posthoc* P-value baseline vs. TP2 | P-value TP1 vs. TP2 |
|---|---|---|---|---|---|---|---|
| MIDAS SCORE | 36.3 (41.9) | 18.3 (38.8) | 16.0 (32.8) | <0.001 | <0.001 | <0.001 | 0.176 |
| MIDAS 1: days missed at work | 7.0 (15.2) | 3.8 (13.6) | 2.6 (10.9) | <0.001 | <0.001 | 0.002 | 0.093 |
| MIDAS 2: days with >50% reduced productivity at work | 7.6 (5.4) | 4.2 (4.6) | 3.9 (4.9) | 0.001 | 0.002 | 0.001 | 0.650 |
| MIDAS 3: days without household work | 6.3 (9.7) | 3.6 (8.1) | 2.6 (4.4) | <0.001 | 0.002 | <0.001 | 0.161 |
| MIDAS 4: days with >50% reduced productivity in household work | 5.8 (6.8) | 3.9 (7.0) | 3.9 (8.2) | 0.006 | <0.009 | 0.046 | 0.766 |
| MIDAS 5: days when family, social or leisure activities are missed | 9.8 (15.3) | 3.7 (9.9) | 3.1 (7.2) | <0.001 | <0.001 | <0.001 | 0.481 |
| MIDAS: Headache days | 26.4 (19.3) | 17.0 (15.5) | 17.0 (18.1) | <0.001 | 0.002 | <0.001 | 0.487 |
| MIDAS: Pain intensity (scale 0–10) | 6.1 (1.7) | 4.8 (1.5) | 4.5 (2.0) | <0.001 | <0.001 | <0.001 | 0.078 |
| MIDAS: Grade | 3.6 (0.7) | 2.4 (1.0) | 2.2 (1.2) | <0.001 | <0.001 | <0.001 | 0.295 |
Baseline: before treatment, TP1: 1–90 days use of desogestrel; TP2: 91–180 days desogestrel use.
*after Bonferroni correction, post-hoc p-values are significant at p < 0.017.
Table 4.
Changes in migraine and headache frequency during use of the progestin-only pill desogestrel 75 μg, comparison between MO (n=32) and MA (10) patients
| MO Mean (SD) baseline | MA Mean (SD) baseline | MO Mean (SD) TP 2 | MA Mean (SD) TP 2 | p-value MO baseline vs. TP 2 | p-value MA baseline vs. TP 2 | |
|---|---|---|---|---|---|---|
| Headache days/month | 4.1 (4.3) | 3.8 (5.2) | 3.4 (4.6) | 1.9 (2.0) | 0.06 | 0.60 |
| Migraine days/month | 5.4 (4.1) | 6.8 (4.8) | 3.2 (3.8) | 4.6 (4.3) | 0.007 | 0.09 |
| Sum headache and migraine/month | 9.5 (5.6) | 8.8 (3.6) | 6.6 (4.4) | 6.6 (4.4) | <0.001 | 0.11 |
| Headache intensity/month | 16.0 (8.3) | 14.4 (5.4) | 11.4(8.4) | 8.5 (6.4) | <0.001 | 0.24 |
| Pain medication | 6.2 (3.6) | 9.8 (9.5) | 4.7 (3.6) | 5.8 (4.8) | 0.03 | 0.03 |
| Triptan use in 3 months | 11.9 (11.8) | 13.1 (22.2) | 8.4 (10.7) | 6.2 (8.6) | 0.005 | 0.02 |
MO = migraine without aura, MA = Migraine with aura, baseline : days 1–90 before treatment; TP 2: days 91–180 of treatment.
Discussion
In the present study we report the effects of 180 days of contraception with the progestin-only pill desogestrel 75 μg on headache and migraine. We observed a significant reduction in migraine frequency, migraine intensity, use of triptans and pain score. Quality of life measured by the MIDAS score improved by more than 50%. Mean MIDAS grades were diminished by point (Table 3). The majority of positive effects were apparent after 90 days and small further improvements were noted up to 180 days of use (Table 2). To our knowledge, we report for the first time that hormonal treatment can reduce the use of triptans significantly. This might be of relevance for women at the boarder of medication overuse headaches. As different pathophysiologies underlie MA and MO, we performed subanalyses for both types of headaches. In women with MO, significant improvements for all variables except headache days were observed. In the group of patients suffering from MA (n = 10) migraine days decreased by two/month, what possibly as a result of the small group size was not significant. Significant bettermends were observed with regard to use of pain medications, use of triptans and MIDAS score and grade.
Migraine is a typical disorder with a high response rate to placebo in controlled trials. For ethical reasons placebo-controlled studies in the area of contraception are not acceptable. An important strength of the present study is the long run-in period and the evaluation not only of migraine frequency but also of additional parameters, like pain intensity, use of pain medications and quality of life. The combination of these data is a better reflection of the overall well-being as demonstrated in the detailed data analysis of the patients developing more migraine in our study. The run in period of 90 days allowed a balanced overview with regard to migraine frequencies which can vary markedly from month to month. However, even if the headache diaries had been conducted prospectively our analyses could have generated selection or information bias. In particular, we assume that a prospective design might have resulted in a higher continuation rate and exclusion of less charts with incomplete diaries. A control group of women using other hormonal contraceptive methods would have been of advantage.
Our findings for MO patients are in accordance with a very recent retrospective diary-based study, demonstrating a significant reduction in migraine frequency, pain intensity and use of pain medication with 6 months use of desogestrel [26]. Triptan use did not decline, which contrasts with our result and might be related to the lower number of included patients. The comparison with a control group of users of a combined pill (COC) in a long-cycle in this study is of great interest, because both forms of contraception prevent hormone withdrawal [26]. While migraine attacks and pain intensity decreased significantly with the POP, headache frequency declined with the COC regimen only. Desogestrel use failed to exert a significant effect on non-migrainous-headache in our sample and the comparative trial. This can be explained, by our earlier reported findings showing in an individual follow-up of both headache and migraine, that a temporary transformation of migraines to headaches occurs in some women [19]. Our present study with a longer observation period however indicates that, on the long-term, these headaches might decline as well (p = 0.05). Although our study includes only few women with MA, the findings are backed by Nappi et al. who reported a significant reduction in migraine frequency in MA patients, but did not investigate pain intensity and quality of life [27].
Even if there is still a lack of prospective controlled trials several diary-based studies indicate a positive impact of desogestrel on migraine without aura [19,26,27]. Continuous use of COCs exerts a positive impact on headaches and hormone-withdrawal migraines, however POP are much safer with regard to the cardiovascular and thromboembolic risks [26,28-32]. The benefit of desogestrel on migraine with aura, which is not typically associated with estrogen withdrawal, has to be confirmed in future studies. Many migraineurs are reluctant to use hormones as a consequence of previous bad experience. During counselling it is helpful to know that major improvements can be expected during 90 days of desogestrel use. Furthermore, two trials indicate that migraines and pain tend to improve further beyond 3 months [19,26,27]. On the other hand, patients have to be informed that migraine rarely worsens. The clinically meaningful 30% reduction in pain (considered by the IHS as clinically meaningful) in 60% of our patients is another argument to prefer this contraceptive method in women with migraine [24,25]. Reduction in use of triptans and other pain medications might contribute of the prevention of medication-overuse headaches.
In daily life the degree to which a reduction in headache frequency translates to decreased disability and improved quality of life is highly relevant. The MIDAS demonstrated highly reduced disability and significantly improved quality of life in our patients.
Use of POP can cause a variety of bleeding patterns including amenorrhoea, infrequent bleeding, frequent bleeding and prolonged bleeding episodes. Unfavorable bleeding patterns such as frequent bleeding and prolonged bleeding occur as result of the continuous progestin effect on the endometrium and can be a reason for withdrawal from this form of contraception [33]. Prolonged and frequent bleedings usually stop with longer duration of use and can be treated if not.
Unanswered questions
New insights in the hormonal effects on the brain allow speculations about mechanisms underlying our observations. Avoidance of hormone withdrawal can only explain the decline of cycle-related headaches. In contrast to estrogens, progesterone seems to attenuate trigeminovascular nociception and reduces dural plasma protein extravasation following stimulation of the trigeminal ganglion [34-36]. Thus direct or receptor-mediated effects of the desogestrel on the trigeminovascular system can be postulated. The variety of responses on desogestrel treatment could be a result of the genetic variability of estrogen receptors in women, with some polymorphisms being a significant risk factor for migraine [37]. The neurological basis of migraine auras has not yet been established, increasing evidence indicates that they are a clinical manifestation of a cortical spreading depression (CSD).
In mice the thresholds for cortical spreading depression (CSD) is lower in cycling females than in males. This would allow to hypothesise that maintenance of low estrogen levels induced by desogestrel might upregulate the threshold for CSD thus reduce MA attacks. A further mechanism could be that desogestrel or its metabolite etonogestrel, like progesterone and allopregnanolone decrease cortical excitability via the GABA -receptor [38-40].
At the moment we have no means to predict how an individual migraineur will react on desogestrel. Outside the study we achieved positive effects with higher dosages. However, this is off-label use and cannot be generally recommended before prospective trials have been conducted. Several trials highlight a positive effect of desogestrel on migraine. Among neurologists it is well known that headaches may be cycle-related, but they rarely consider to search advice for a hormonal treatment. Vice versa gynaecologists are not always aware of the fact that hormonal treatment affects headache frequency in predisposed women. A closer collaboration between gynaecologic endocrinologists and headache specialists might provide better care and safety for young women, suffering from migraine during use of any hormones or in association with their natural cycle.
Conclusion
In conclusion our data indicate a positive impact of desogestrel 75 μg on migraine frequency, intensity, use of pain medication and quality of life. The major improvement was observed during the initial 90 days of use, which might be important for patients’ counselling. Randomised controlled trials are needed to substantiate our results.
Acknowledgements
The authors declare that they received not any funding for the study or for preparing the manuscript with the exception of the salaries from their institutions.
Funding
This research received no specific grant from any funding agency in the public, commercial or for non–profit sectors.
Footnotes
Competing interests
Gabriele S Merki-Feld and Bruno Imthurn had financial relationship (lecturer, member of advisory boards and// or consultant) with Bayer-Schering Pharma and MSD AG.
RL and AG declared no conflicts of interest.
Authors’ contributions
GM: participated in the design of the study, the acquisition and analysis of data and the drafting of the manuscript. BI: has been involved in drafting the manuscript and revising it critically. RL participated in the acquisition and interpretation of data and revision of the manuscript. BS has been involved in the analysis and interpretation of data and revision of the manuscript. AG participated in the design of the study, acquisition of data and drafting the manuscript. All authors read and approved the final version of the manuscript.
Contributor Information
Gabriele S Merki-Feld, Email: gabriele.merki@usz.ch.
Bruno Imthurn, Email: bruno.imthurn@sz.ch.
Ronald Langner, Email: ronald.langner@kopfwww.ch.
Burkhardt Seifert, Email: seifert@ifspm.uzh.ch.
Andreas R Gantenbein, Email: a.gantenbein@rehaclinic.ch.
References
- 1.Aegidius K, Zwart JA, Hagen K, Schei B, Stovner LJ. Oral contraceptives and increased headache prevalence: the Head-HUNT Study. Neurology. 2006;66(3):349–353. doi: 10.1212/01.wnl.0000196481.57994.09. [DOI] [PubMed] [Google Scholar]
- 2.Allais G, Gabellari IC, Airola G, Borgogno P, Schiapparelli P, Benedetto C. Headache induced by the use of combined oral contraceptives. Neurol Sci. 2009;30(Suppl 1):S15–S17. doi: 10.1007/s10072-009-0051-9. [DOI] [PubMed] [Google Scholar]
- 3.Teepker M, Peters M, Kundermann B, Vedder H, Schepelmann K, Lautenbacher S. The effects of oral contraceptives on detection and pain thresholds as well as headache intensity during menstrual cycle in migraine. Headache. 2011;51(1):92–104. doi: 10.1111/j.1526-4610.2010.01775.x. [DOI] [PubMed] [Google Scholar]
- 4.Loder EW, Buse DC, Golub JR. Headache as a side effect of combination estrogen-progestin oral contraceptives: a systematic review. Am J Obstet Gynecol. 2005;193(3 Pt 1):636–649. doi: 10.1016/j.ajog.2004.12.089. [DOI] [PubMed] [Google Scholar]
- 5.Archer DF, Jensen JT, Johnson JV, Borisute H, Grubb GS, Constantine GD. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74(6):439–445. doi: 10.1016/j.contraception.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Macgregor EA. Menstrual migraine: a clinical review. J Fam Plann Reprod Health Care. 2007;33(1):36–47. doi: 10.1783/147118907779399684. [DOI] [PubMed] [Google Scholar]
- 7.Macgregor EA, Rosenberg JD, Kurth T. Sex-related differences in epidemiological and clinic-based headache studies. Headache. 2011;51(6):843–859. doi: 10.1111/j.1526-4610.2011.01904.x. [DOI] [PubMed] [Google Scholar]
- 8.Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case–control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318(7175):13–18. doi: 10.1136/bmj.318.7175.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bigal ME, Kurth T, Santanello N, Buse D, Golden W, Robbins M, Lipton RB. Migraine and cardiovascular disease: a population-based study. Neurology. 2010;74(8):628–635. doi: 10.1212/WNL.0b013e3181d0cc8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzourio C, Tehindrazanarivelo A, Iglesias S, Alperovitch A, Chedru F, D’Anglejan-Chatillon J, Bouser MG. Case–control study of migraine and risk of ischaemic stroke in young women. BMJ. 1995;310(6983):830–833. doi: 10.1136/bmj.310.6983.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schurks M, Buring JE, Kurth T. Migraine, migraine features, and cardiovascular disease. Headache. 2010;50(6):1031–1040. doi: 10.1111/j.1526-4610.2009.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzourio C, Iglesias S, Hubert JB, Visy JM, Alperovitch A, Tehindrazanarivelo A, Biousse V, Woimant F, Bousser MG. Migraine and risk of ischaemic stroke: a case–control study. BMJ. 1993;307(6899):289–292. doi: 10.1136/bmj.307.6899.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: A meta-analysis. JAMA. 2000;284(1):72–78. doi: 10.1001/jama.284.1.72. [DOI] [PubMed] [Google Scholar]
- 14.Sacco S, Ricci S, Degan D, Carolei A. Migraine in women: the role of hormones and their impact on vascular diseases. J Headache Pain. 2012;13(3):177–189. doi: 10.1007/s10194-012-0424-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lidegaard O, Lokkegaard E, Svendsen AL, Agger C. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890. doi: 10.1136/bmj.b2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nappi RE, Merki-Feld GS, Terreno E, Pellegrinelli A, Viana M. Hormonal contraception in women with migraine: is progestogen-only contraception a better choice? J Headache Pain. 2013;14:66. doi: 10.1186/1129-2377-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barkfeldt J, Virkkunen A, Dieben T. The effects of two progestogen-only pills containing either desogestrel (75 microg/day) or levonorgestrel (30 microg/day) on lipid metabolism. Contraception. 2001;64(5):295–299. doi: 10.1016/S0010-7824(01)00269-4. [DOI] [PubMed] [Google Scholar]
- 18.Kivela A, Ruuskanen M, Agren U, Dieben T. The effects of two progrestogen-only pills containing either desogestrel (75 microgram/day) or levonorgestrel (30 microgram/day) on carbohydrate metabolism and adrenal and thyroid function. Eur J Contracept Reprod Health Care. 2001;6(2):71–77. doi: 10.1080/ejc.6.2.71.77. [DOI] [PubMed] [Google Scholar]
- 19.Merki-Feld GS, Imthurn B, Langner R, Sandor PS, Gantenbein AR. Headache frequency and intensity in female migraineurs using desogestrel-only contraception: a retrospective pilot diary study. Cephalalgia. 2013;33(5):340–346. doi: 10.1177/0333102412473373. [DOI] [PubMed] [Google Scholar]
- 20.Merki-Feld GS, Imthurn B, Seifert B, Merki LL, Agosti R, Gantenbein AR. Desogestrel-only contraception reduces headache frequency and improves quality of life in female migraineurs. Eur J Contracept Reprod Health Care. 2013;18:394–400. doi: 10.3109/13625187.2013.814769. [DOI] [PubMed] [Google Scholar]
- 21.IHS International Headache Society The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24 Suppl 1:9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 22.Stewart WF, Lipton RB, Kolodner KB, Sawyer J, Lee C, Liberman JN. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain. 2000;88(1):41–52. doi: 10.1016/S0304-3959(00)00305-5. [DOI] [PubMed] [Google Scholar]
- 23.Lipton RB, Stewart WF, Sawyer J, Edmeads JG. Clinical utility of an instrument assessing migraine disability: the Migraine Disability Assessment (MIDAS) questionnaire. Headache. 2001;41(9):854–861. doi: 10.1046/j.1526-4610.2001.01156.x. [DOI] [PubMed] [Google Scholar]
- 24.Silberstein S, Tfelt-Hansen P, Dodick DW, Limmroth V, Lipton RB, Pascual J, Wang SJ. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia. 2008;28(5):484–495. doi: 10.1111/j.1468-2982.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- 25.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Hertz S, Heyse JF, Iyengar S, Jadad AR, Jay GW, Jermano JA, Katz NP, Manning DC, Martin S, Max MB, McGrath P, McQuay HJ, Quessy S, Rappaport BA, Revicki DA, Rothman M, Stauffer JW, Svensson O, White RE, Witter J. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Morotti M, Remorgida V, Venturini PL, Ferrero S. Progestin-only contraception compared with extended combined oral contraceptive in women with migraine without aura: a retrospective pilot study. Eur J Obstet Gynecol Reprod Biol. 2014;183:178–182. doi: 10.1016/j.ejogrb.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 27.Nappi RE, Sances G, Allais G, Terreno E, Benedetto C, Vaccaro V, Facchinetti F. Effects of an estrogen-free, desogestrel-containing oral contraceptive in women with migraine with aura: a prospective diary-based pilot study. Contraception. 2011;83(3):223–228. doi: 10.1016/j.contraception.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 28.Calhoun A, Ford S, Pruitt A. The impact of extended-cycle vaginal ring contraception on migraine aura: a retrospective case series. Headache. 2012;52(8):1246–1253. doi: 10.1111/j.1526-4610.2012.02211.x. [DOI] [PubMed] [Google Scholar]
- 29.De Leo V, Scolaro V, Musacchio MC, Di Sabatino A, Morgante G, Cianci A. Combined oral contraceptives in women with menstrual migraine without aura. Fertil Steril. 2011;96(4):917–920. doi: 10.1016/j.fertnstert.2011.07.1089. [DOI] [PubMed] [Google Scholar]
- 30.Sulak P, Willis S, Kuehl T, Coffee A, Clark J. Headaches and oral contraceptives: impact of eliminating the standard 7-day placebo interval. Headache. 2007;47(1):27–37. doi: 10.1111/j.1526-4610.2007.00650.x. [DOI] [PubMed] [Google Scholar]
- 31.MacGregor EA, Guillebaud J. Combined oral contraceptives, migraine and ischaemic stroke. Clinical and Scientific Committee of the Faculty of Family Planning and Reproductive Health Care and the Family Planning Association. Br J Fam Plann. 1998;24(2):55–60. [PubMed] [Google Scholar]
- 32.Lidegaard O, Nielsen LH, Skovlund CW, Lokkegaard E. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001–10. BMJ. 2012;344:e2990. doi: 10.1136/bmj.e2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merki-Feld GS, Breitschmid N, Seifert B, Kreft M. A survey on Swiss women’s preferred menstrual/withdrawal bleeding pattern over different phases of reproductive life and with use of hormonal contraception. Eur J Contracept Reprod Health Care. 2014;19(4):266–275. doi: 10.3109/13625187.2014.907398. [DOI] [PubMed] [Google Scholar]
- 34.Cutrer FM, Moskowitz MA. Wolff Award 1996. The actions of valproate and neurosteroids in a model of trigeminal pain. Headache. 1996;36(10):579–585. doi: 10.1046/j.1526-4610.1996.3610579.x. [DOI] [PubMed] [Google Scholar]
- 35.Multon S, Pardutz A, Mosen J, Hua MT, Defays C, Honda S, Harada N, Bohotin C, Franzen R, Schoenen J. Lack of estrogen increases pain in the trigeminal formalin model: a behavioural and immunocytochemical study of transgenic ArKO mice. Pain. 2005;114(1–2):257–265. doi: 10.1016/j.pain.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 36.Bolay H, Berman NE, Akcali D. Sex-related differences in animal models of migraine headache. Headache. 2011;51(6):891–904. doi: 10.1111/j.1526-4610.2011.01903.x. [DOI] [PubMed] [Google Scholar]
- 37.Joshi G, Pradhan S, Mittal B. Role of the oestrogen receptor (ESR1 PvuII and ESR1 325 C- > G) and progesterone receptor (PROGINS) polymorphisms in genetic susceptibility to migraine in a North Indian population. Cephalalgia. 2010;30(3):311–320. doi: 10.1111/j.1468-2982.2009.01967.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu A, Margaill I, Zhang S, Labombarda F, Coqueran B, Delespierre B, Marchand-Leroux C, O’Malley BW, Lydon JP, De Nicola AF, Sitruk-Ware R, Mattern C, Plotkine M, Schumacher M, Guennoun R. Progesterone receptors: a key for neuroprotection in experimental stroke. Endocrinology. 2012;153(8):3747–3757. doi: 10.1210/en.2012-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schumacher M, Mattern C, Ghoumari A, Oudinet JP, Liere P, Labombarda F, Sitruk-Ware R, De Nicola AF, Guennoun R. Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors. Prog Neurobiol. 2013;113:6–39. doi: 10.1016/j.pneurobio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with gamma-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270(3):1223–1229. [PubMed] [Google Scholar]


