Abstract
The regulation of vascular resistance in fishes has largely been studied using isolated large conductance vessels, yet changes in tissue perfusion/vascular resistance are primarily mediated by the dilation/constriction of small arterioles. Thus we adapted mammalian isolated microvessel techniques for use in fish and examined how several agents affected the tone/resistance of isolated coronary arterioles (<150 μm ID) from steelhead trout (Oncorhynchus mykiss) acclimated to 1, 5, and 10°C. At 10°C, the vessels showed a concentration-dependent dilation to adenosine (ADE; 61 ± 8%), sodium nitroprusside (SNP; 35 ± 10%), and serotonin (SER; 27 ± 2%) (all values maximum responses). A biphasic response (mild contraction then dilation) was observed in vessels exposed to increasing concentrations of epinephrine (EPI; 34 ± 9% dilation) and norepinephrine (NE; 32 ± 7% dilation), whereas the effect was less pronounced with bradykinin (BK; 12.5 ± 3.5% constriction vs. 6 ± 6% dilation). Finally, a mild constriction was observed after exposure to acetylcholine (ACh; 6.5 ± 1.4%), while endothelin (ET)-1 caused a strong dose-dependent increase in tone (79 ± 5% constriction). Acclimation temperature had varying effects on the responsiveness of vessels. The dilations induced by EPI, ADE, SER, and SNP were reduced/eliminated at 5°C and/or 1°C as compared with 10°C. In contrast, acclimation to 5 and 1°C increased the maximum constriction induced by ACh and the sensitivity of vessels to ET-1 (but not the maximum response) at 1°C was greater. Acclimation temperature had no effect on the response to NE, and responsiveness to BK was variable.
Keywords: microvessels, coronary, vasomotor responses, cold, hormones, paracrine effects, endothelium, vascular tone, vascular resistance
fish show considerable variation with respect to development of the coronary circulation. For example, only about 30% of all fish (i.e., all elasmobranchs and approximately one-third of teleosts) have a coronary circulation, and when present, the coronary vasculature can vary from exclusively perfusing the compact myocardium of the ventricle, to supplying both the compact and spongy myocardium of the ventricle with blood, to perfusing both the atrial and ventricular myocardium (21). Over the past 25 years we have learned a reasonable amount about the importance of coronary blood flow (qCor) for fish cardiac performance, and how perfusion of this vascular bed is controlled. Unlike the mammalian heart, many fish that possess a coronary circulation (e.g., salmonids and eels) are able to survive without it, and their cardiac performance at rest is, for most part, unaffected by ablation/ligation of the main coronary vessel (14, 22, 36). Nonetheless, some cardiovascular compensatory mechanisms are observed following ablation of the coronary artery (including an increase in heart rate, a decrease in blood pressure, and regrowth of the coronary artery) (15, 22, 27), and it is clear that the coronary circulation is important in situations where oxygen demand and cardiac function increase. For example, acute coronary ligation reduced the maximal prolonged swimming speed of chinook salmon (Oncorhynchus tshawytscha) by 32% (22) and increased the length of recovery from a swimming challenge in rainbow trout (95). Furthermore, qCor can increase by almost threefold with increased activity and/or exposure to low oxygen levels (5, 37). In contrast, it seems that type IV hearts (e.g., those found in tunas and their relatives) are highly dependent on the presence of an intact coronary circulation, as perfusion of the heart lumen with oxygenated saline is insufficient to support resting cardiac performance (14, 20, 28).
qCor is primarily determined by changes in coronary vascular resistance/tone and alterations in dorsal aortic blood pressure (21). Analysis of the pressure-flow relationships for trout coronaries (19) and of the relationship between cardiac power output and qCor, in both salmon (Oncorhynchus kisutch) and trout (5, 37), have shown that small increases in dorsal aortic blood pressure (e.g., due to activity) can lead to large increases in qCor. This means that a large coronary vasodilator reserve exists, and that under resting conditions there is a tonic vasoconstriction of the coronary circulation that can be released to increase coronary flow (21, 25). Experiments examining various aspects of fish vascular (including coronary) physiology have been primarily restricted to large (conductance) vessels (47, 74, 76, 93). However, contradictory results emerge when comparing studies where the trout coronary circulation is perfused versus studies where the responses of vascular rings are examined (e.g., see Refs. 1, 68, 69 vs. 92, 93). This is likely due to a number of factors. First, there is considerable heterogeneity in how vessels of various sizes respond to important hormones and other vasoactive agents (49, 104), and changes in tissue perfusion/vascular resistance are primarily mediated by the dilation or constriction of vessels <150 μm in diameter (8, 17, 53). These small arteries and arterioles are commonly referred to as resistance vessels (or microvessels) and in fish, as in mammals, it is assumed that most of the control of blood flow resides in these vessels (75) and not in conductance vessels as have been previously examined. Second, microvessel tone is both remotely (through neural and hormonal mechanisms) and locally controlled (30, 75); the local system adjusting blood flow to match the metabolic demand of a particular tissue (4, 75).
Mammalian researchers have been using isolated microvessel techniques for over 30 years to directly examine how numerous parameters affect the dilation and constriction of microvessels (17, 48, 53), and thus, how qCor is controlled. Therefore, the goals of our research were to: 1) adapt these techniques for the study of fish vessels; and 2) examine how various components of both the remote and local systems affect fish coronary microvessel tone/resistance. Moreover, given that temperature has a profound effect on numerous aspects of cardiac metabolic demand and perfusion (35), and that no data exist on the effects of temperature on qCor or resistance, these experiments were performed at several acclimation temperatures (1, 5, and 10°C). These temperatures were chosen because we know very little about the cardiovascular physiology of nonpolar species at cold temperatures, and this is presently a focus in our laboratory (e.g., 59, Costa IASF, Hein TW, Secombes CJ, and Gamperl AK; unpublished observations).
MATERIALS AND METHODS
Experimental Animals
Adult steelhead trout (Oncorhynchus mykiss) were purchased from a cage-culture site on the south coast of Newfoundland (Canada) and initially held at 10 ± 1.0°C in three 6-m3 tanks at the Dr. Joe Brown Aquatic Research Building (JBARB) (Dept. of Ocean Sciences, Memorial University of Newfoundland). Fish were then acclimated to three different temperatures (1, 5, and 10 ± 1.0°C) for a minimum of 5 wk before experiments began. Fish were fed a maintenance ration (1.5% per body mass every 2 days) of commercial pellets (Skretting, Vancouver, BC, Canada) while held at the JBARB and were maintained on a 12-h light:12-h dark photoperiod.
All procedures involving these fish followed the guidelines published by the Canadian Council on Animal Care and were approved by Memorial University of Newfoundland's Institutional Animal Care Committee.
Sampling of Heart Tissue
Fish were euthanized by a sharp blow to the head, and the heart was quickly removed and placed in ice-chilled 0.9% NaCl. The bulbus and the atrium were then removed, and the ventricle was transferred to a temperature-controlled dissection bath filled with cooled (0–1°C or 4 ± 0.5°C) physiological saline solution. The ventricle was then cut in half under a dissecting microscope (model MZ9s; Leica Microsystems, Concord, Canada) to allow for dissection of the arterioles (microvessels). The physiological saline solution contained (in mM) 155.0 NaCl, 4.7 KCl, 2.5 CaCl2·2H2O, 1.99 MgSO4·7H2O, 3.0 HEPES acid, 7.0 HEPES sodium salt, 1.0 NaH2PO4·H2O, and 5.6 glucose. This saline, as well as the high KCl and zero calcium physiological saline solutions (see Isolation and Cannulation of Coronary Arterioles and Experimental Setup and Protocol, respectively), were buffered to a pH of 7.8 at 10°C and filtered through a 0.22-μm cellulose acetate, low-protein binding, membrane (Corning 430521, Corning, Corning, NY).
Steelhead trout possess a type II heart with an outer compact myocardium separated from the spongy myocardium by a layer of connective tissue. In this species the coronary vessels do not penetrate the spongy myocardium, and the coronary circulation empties directly into the ventricular lumen (14, 21, 101). This simplifies coronary microvessel dissection, with no need to distinguish between arterioles and venules or to fill vessels with a contrasting solution (e.g., physiological saline containing India ink and gelatin) before dissection.
Isolation and Cannulation of Coronary Arterioles
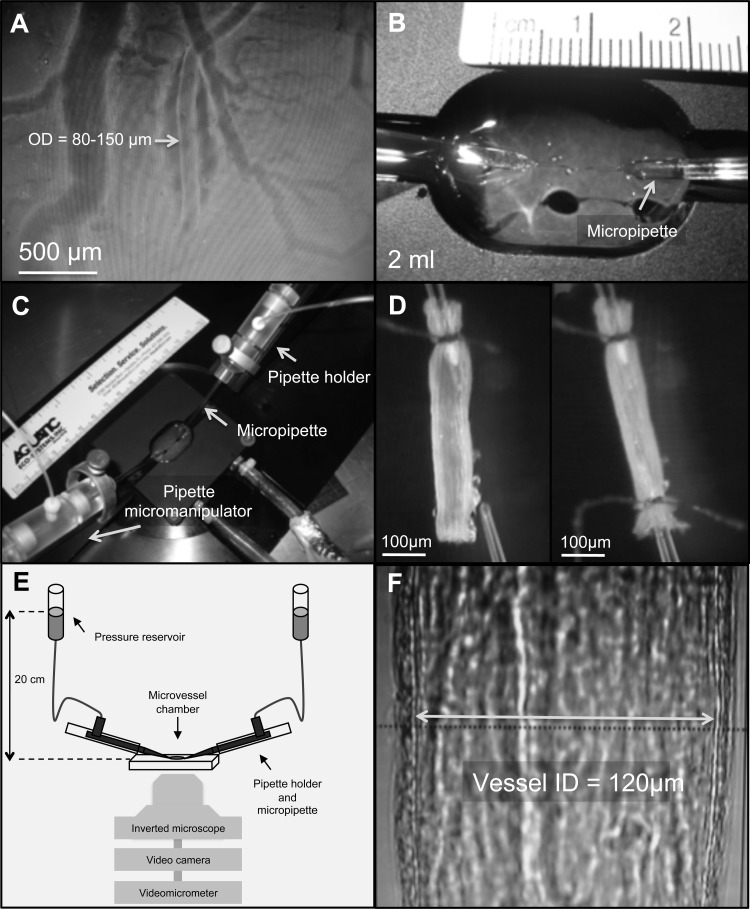
With the aid of a dissecting microscope (model MZ9s), an unbranched arteriole of ∼80–150 μm external diameter and 1 to 1.5 mm long was selected and carefully dissected free from the surface of the ventricle (Fig. 1A) using pairs of specially sharpened Dumont (no. 5, mirror finished) forceps and Vannas spring scissors (straight; 4 mm cutting edge; Fine Science Tools, Foster City, CA). After careful removal of any remaining extraneous tissue, the arteriole was transferred for cannulation to a custom-made (Technical Services, Memorial University of Newfoundland) vessel bath (volume = 2 ml; see Fig. 1B) containing physiological saline solution at the acclimation temperature (1, 5, or 10°C). This vessel bath was made of titanium (to resist corrosion and ensure efficient heat transfer) and equipped with a water jacket that allowed temperature control of the saline solution within the bath throughout the experiment. The temperature of the saline within the vessel bath was maintained by connecting the water jacket to a recirculating water bath (Isotemp model 3016S, Thermo Fisher Scientific, Waltham, MA). The vessel chamber was, in turn, attached to a specially designed stage (Cardiovascular Research Institute, Texas A & M) equipped with micromanipulators (model MMN-1, Narishige, East Meadow, NY) that housed the pipette holders and allowed for adjustments in pipette positioning in all three dimensions (Fig. 1C).
Fig. 1.
Isolation and cannulation of coronary microvessels, and methods used to study their vasoactivity. A: an unbranched arteriole [∼80–150 μm outside diameter (OD) and 1–1.5 mm long] on the surface of the ventricle. B: custom-made vessel bath containing saline solution at the experimental temperature (1°C, 5°C, or 10°C). C: vessel chamber is attached to a specially designed stage equipped with micromanipulators that house pipette holders and allowed for adjustments in pipette positioning in all three dimensions. D: arteriole was cannulated by pulling one end of the vessel onto the tip of an angled glass micropipette filled with saline (with 1% albumin) and securely tied to the pipette. After any remaining blood was flushed out, the other end of the vessel was cannulated using a second micropipette and tied in place. E and F: vessel stage was then transferred to an inverted microscope coupled with a video camera and videomicrometer system for continuous measurement of the vessel internal diameter.
The arteriole was cannulated by pulling one end of the vessel onto the tip (outer diameter of 30–40 μm) of an angled (∼30°) glass micropipette (B200-116-10, Sutter Instruments, Novato, CA) filled with saline containing 1% albumin and securely tying it to the pipette with 11-0 ophthalmic suture (Alcon, Fort Worth, TX). Any remaining blood was then flushed out of the vessel at low perfusion pressure (10 cmH2O or ∼7.5 mmHg), and the other end of the vessel was cannulated using a second micropipette and tied in place (Fig. 1D). The whole procedure, from the start of arteriole dissection to the end of cannulation, took ∼30 min.
After cannulation was complete, the vessel stage was transferred from the dissecting microscope to an inverted microscope (model DM1RB, Leica) with a ×20 objective, coupled to a video camera (Sony CCD-Iris; Sony) and videomicrometer system (model VIA-100; Boeckeler Instruments, Tuscon, AZ) for continuous measurement of vessel internal diameter (Fig. 1, E and F). At this point, the glass pipettes were connected to independent adjustable pressure reservoirs filled with physiological saline solution, and pressure in the vessels was set to 20 cmH2O (∼15 mmHg) luminal pressure, without flow, by adjusting the height of the reservoirs. This pressure approximates the in vivo intraluminal pressure of trout coronary microvessels of this size range: 1) mammalian studies have estimated that the intraluminal pressure of coronary arterioles in this size range is ∼50% of mean aortic pressure (11, 53); and 2) values for dorsal aortic blood pressure in rainbow trout at rest range from 38.8 to 42 cmH2O (∼28.5 to 31 mmHg) (37, 77, 86). The vessel was then set to its resting in situ length (as measured with an eyepiece micrometer before dissection) by stretching the vessel using the pipette micromanipulators and tested for leaks (any preparations with leaks were excluded from further investigation). At this point, the bath solution was replaced with fresh physiological saline and the vessel allowed ∼30 min to develop basal tone. However, vessels that did not regain tone (see results for the percentage of vessels) were precontracted by replacing the physiological saline in the bath with physiological saline solution containing 50 mM KCl: the exact composition of this saline (in mM) was the following: 110.0 NaCl, 50.0 KCl, 2.5 CaCl2·2H2O, 1.99 MgSO4·7H2O, 3.0 HEPES acid, 7.0 HEPES sodium salt, 1.0 NaH2PO4·H2O, and 5.6 glucose. Preliminary experiments showed that this concentration of KCl produced a constriction of 60 to 70% of maximum diameter within 15–20 min, and that this tone could be maintained for at least 120 min (data not shown). This degree of constriction was similar to that observed for trout coronary arterioles that developed basal tone and is considered in mammalian studies to be an appropriate level of basal tone for similar size arterioles (42, 53, 54). Furthermore, statistical analysis did not detect a difference in the vasoactive responses of vessels that developed spontaneous tone versus those preconstricted with KCl.
Experimental Setup and Protocol
Dose-response curves.
The effect of several pharmacological agents (see Vasoactive agents) on vessel vasoactivity was assessed by exposing the vessel to increasing concentrations of a particular agent and measuring changes in the vessel's internal diameter (ID). Each agent was injected into the vessel bath in increments of 20 μl to achieve the desired concentrations.
Changes in vessel ID were measured 10 min after injection, and the next amount of agonist was added to the bath immediately thereafter. Once the final measurement for a particular agent was completed, the saline in the vessel bath was exchanged three times to remove any residual amounts. Up to four different agents were tested on a single vessel. However, several precautions were taken to ensure the consistency and accuracy of results: 1) repeated injections of saline into the bath were performed in several preliminary experiments, and dilation/constriction was never observed (data not shown); 2) when using both dilatory and constrictor agents on a single vessel, agents causing vasodilation were used first; 3) a subsequent agent was only tested if the vessel returned to its resting diameter after the previous agent was washed out; and 4) although strong and/or long-lasting vasodilators or vasoconstrictors were only tested as the last agent for a particular vessel, the degree of dilation/constriction was not different from that seen in preliminary experiments using these agents alone.
After the effects of the last agent were measured on a particular vessel, the vessel was washed three times with physiological saline. The saline in the bath was then replaced with physiological saline without calcium (i.e., ∼0 mM vs. the normal 2.5 mM), and a single injection of adenosine (ADE, final bath concentration 10−4 M) was given to obtain the maximum vessel diameter. ADE was used due to its very strong vasodilator effect on trout coronary arterioles. The time required to obtain the maximum diameter of vessels was variable (20 to 40 min) depending on the last vasoactive agent used. The zero calcium physiological saline solution consisted of (in mM) the following: 155.0 NaCl, 4.7 KCl, 1.99 MgSO4·7H2O, 3.0 HEPES acid, 7.0 HEPES sodium salt, 1.0 NaH2PO4·H2O, and 5.6 glucose.
Vasoactive agents.
The vasoactive agents used in this study were all purchased from Sigma-Aldrich (Oakville ON, Canada): adrenoreceptor agonists epinephrine (EPI) and norepinephrine (NE); purinergic agonist ADE; serotonergic agonist serotonin (SER); cholinergic agonist acetylcholine (ACh); vasoactive peptide bradykinin (BK); endothelin 1 (ET-1), which is a peptide secreted by the vascular endothelium and known potent vasoconstrictor; and sodium nitroprusside (SNP), which is a nitric oxide (NO) donor that acts directly on the vascular smooth muscle. All agents were dissolved in the basic saline solution, except for ET-1 (which was dissolved in deionized water), and kept on ice until needed.
Data Analysis
All measurements of internal diameter were normalized to the resting diameter of vessels at 20 cmH2O luminal pressure (with basal tone or preconstricted with 50 mM KCl). A repeated-measures two-way ANOVA (main effects = agent concentration and acclimation temperature), followed by multicomparisons testing (Tukey's post hoc tests), was used to examine differences in vessel ID due to agent concentration and/or acclimation temperature. When the interaction between the two explanatory variables was significant, separate repeated-measures one-way ANOVAs were performed to test for differences in vessel ID due to vasoactive agent concentration at each acclimation temperature, and one-way ANOVAs were performed at each vasoactive agent concentration. Both of these analyses were followed by Tukey's post hoc tests to identify differences between groups. All statistical analyses were performed using Sigma Plot 12.0 (Systat Software, San Jose, CA) with P < 0.05 set as the level of statistical significance. All values presented in the text, tables, and figures are expressed as means ± SE.
RESULTS
Of all the vessels used in this study (n = 52), 33% developed basal tone and usually within 30 min after cannulation and exposure to intraluminal pressure. Although it was not possible to assess statistically whether temperature had an effect on basal tone, ∼44 and 39% of coronary arterioles from trout acclimated to 1°C and 10°C, respectively, developed spontaneous tone (65–70% ID of resting diameter), whereas only ∼17% of vessels developed basal tone at 5°C. Vessels that developed spontaneous tone normally maintained it throughout the experiment. In contrast, some vessels that developed spontaneous tone following cannulation lost it after the first saline wash (i.e., after exposure to the first vasoactive agent). At this point, these vessels were preconstricted with physiological saline containing 50 mM KCl. Preliminary experiments with vessels (n = 6) preconstricted with physiological saline containing 50 mM KCl showed that they remained constricted for at least 120 min (data not shown). Similarly, to examine the effect of repeated saline injections (i.e., to mimic the injection of vasoactive agents) on vessel tone a series of sham injections (20 μl of saline solution; no agent) was performed (n = 4; data not shown). No significant changes in resting ID were seen after this series of saline injections. Furthermore, the average change from resting diameter (either dilation or constriction) was never more than 0.6%. This effect was not considered significant.
The number of vessels used to test each vasoactive agent at the different acclimation temperatures, their average resting and maximal internal diameters, as well as the general response of vessels to the different agents, are summarized in Table 1.
Table 1.
Summary of the vasomotor responses of isolated trout coronary microvessels to increasing concentrations of several vasoactive agents
| Vasoactive Agent | N | Dose Dependency | Temperature Effect | Acclimation Temperature, °C | Resting ID, μm | Maximal ID, μm | Maximal Change from Resting ID, % |
|---|---|---|---|---|---|---|---|
| EPI | 8 | Constriction/Dilation | Yes; weaker at 1°C | 1 | 78.8 | 110.9 | 12 (+) |
| 5 | 66.0 | 90.7 | 28 (+) | ||||
| 10 | 83.1 | 139.6 | 34 (+) | ||||
| NE | 8 | Constriction/Dilation | No | 1 | 74.3 | 102.2 | 19 (+) |
| 5 | 65.0 | 94.1 | 27 (+) | ||||
| 10 | 94.2 | 145.5 | 32 (+) | ||||
| ADE | 8 (1°C & 5°C)/10 (10°C) | Dilation | Yes; weaker at 5°C and 1°C | 1 | 70.3 | 108.1 | 52 (+) |
| 5 | 84.9 | 117.8 | 42 (+) | ||||
| 10 | 86.8 | 140.4 | 61 (+) | ||||
| SER | 8 | Constriction/Dilation | Yes; weaker at 5°C and 1°C | 1 | 77.4 | 110.9 | 12 (+) |
| 5 | 91.3 | 125.8 | 13 (+) | ||||
| 10 | 87.9 | 126.2 | 27 (+) | ||||
| ACh | 8 | Constriction | Yes; stronger at 5°C and 1°C | 1 | 75.8 | 110.9 | 14 (−) |
| 5 | 60.5 | 96.3 | 18 (−) | ||||
| 10 | 78.8 | 142.4 | 7 (−) | ||||
| BK | 8 | Constriction/Dilation | Yes; variable | 1 | 74.9 | 105.1 | 9 (+) |
| 5 | 64.4 | 106.3 | 10 (−) | ||||
| 10 | 85.8 | 118.7 | 12 (−)/6 (+) | ||||
| ET-1 | 8 | Constriction | Yes; more sensitive at 1°C | 1 | 85.4 | 102.2 | 81 (−) |
| 5 | 67.7 | 102.0 | 84 (−) | ||||
| 10 | 85.4 | 119.8 | 79 (−) | ||||
| SNP | 8 | Constriction/Dilation | Yes; variable | 1 | 80.7 | 102.2 | 8 (+) |
| 5 | 66.8 | 84.1 | 8 (−) | ||||
| 10 | 83.7 | 130.7 | 35 (+) |
Values represent the vasomotor responses of isolated trout coronary microvessels to increasing concentrations of several vasoactive agents at 3 acclimation/test temperatures (1°C, 5°C, and 10°C). EPI, epinephrine; NE, norepinephrine; ADE, adenosine; SER, serotonin; ACh, acetylcholine; BK, bradykinin; ET-1, Endothelin-1; SNP, sodium nitroprusside. The average resting and maximal internal diameter (ID), the general response(s) of the vessels to increasing agent concentration and acclimation temperature, and the maximal change from resting ID [dilation (+), constriction (−)] are shown for each agent.
Catecholamines
Epinephrine and norepinephrine.
Both EPI and NE caused a slight constriction (3% to 13%) of the arterioles at low concentrations (10−9 to 10−7 M), followed by dilation at concentrations of 10−6 M and higher. While vessels at 5°C and 10°C dilated by 28 and 34% of their resting ID following exposure to 10−4 M EPI, respectively, vessels at 1°C only showed a dilation of 12% (Fig. 2A). A similar trend was observed in the vessel response to NE, but these differences were not significant (Fig. 2B).
Fig. 2.
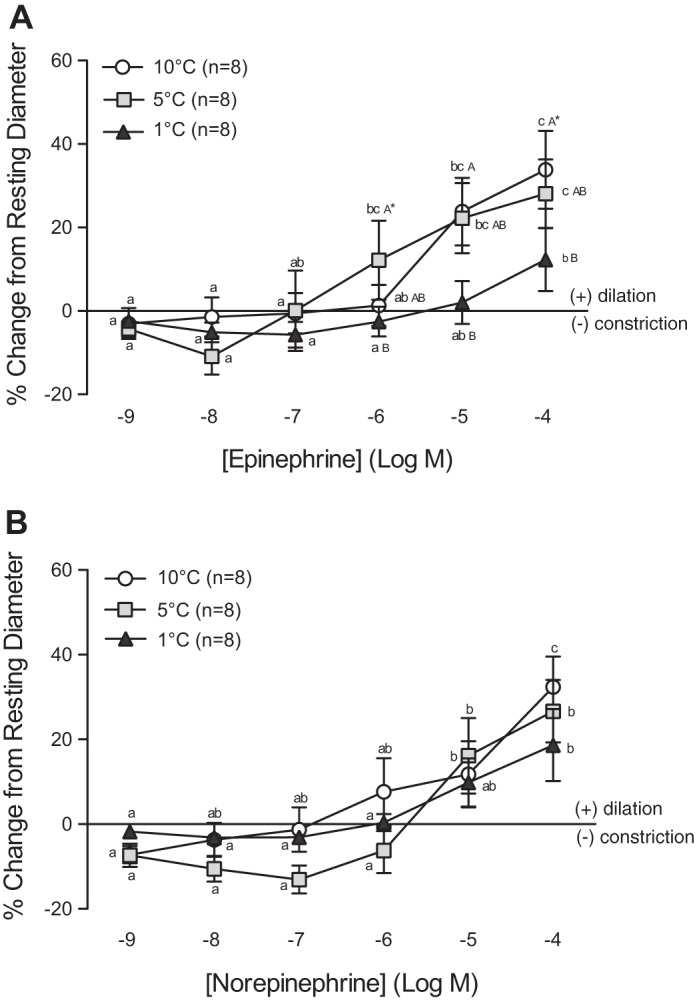
Vasomotor responses of trout coronary arterioles to increasing concentrations of epinephrine (EPI) (A) and norepinephrine (NE) (B) at three acclimation temperatures (1°C, 5°C, and 10°C). Dissimilar lower-case letters indicate significant differences in the response of vessels to the various concentrations of EPI or NE at a given acclimation temperature. Dissimilar capital letters indicate significant differences in the response of vessels at different acclimation temperatures to the same concentration of EPI or NE. To improve clarity of the figure, capital letters are not included when differences were not significant (i.e., AAA). P < 0.05, except where indicated (*), where 0.1 > P ≥ 0.05. Note: a series of sham saline injections did not result in noticeable (i.e., <0.6%) changes in vessel internal diameter (ID). Thus we considered any change in vessel ID greater than the mean change + 2 SD caused by the sham saline injections (∼3%) to be biologically significant.
Adenosine and serotonin.
ADE caused a concentration-dependent dilation of the coronary arterioles over the range of concentrations used in this study (10−9 to 10−4 M) and had the strongest vasodilator effect of all the agents (Fig. 3A). Vessels at 10°C showed an increase in ID of ∼60% when exposed to 10−4 M of ADE. A strong temperature effect was observed in the response of vessels to ADE between 10−6 and 10−4 M, with the dilation of vessels from fish acclimated to 10°C consistently higher (by 1.2- to 2.5-fold) compared with that of vessels from fish acclimated to either 1°C or 5°C (Fig. 3A). At 10−6 and 10−5 M of ADE, the difference in the amount of dilation between vessels from 1°C and 10°C acclimated fish was significant at P < 0.10.
Fig. 3.
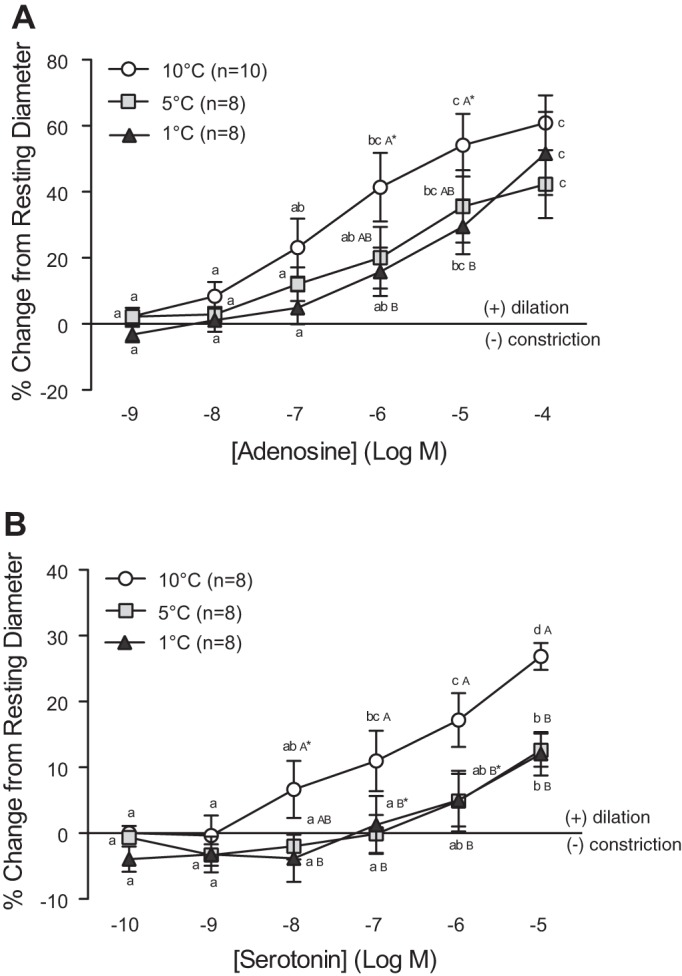
Vasomotor responses of trout coronary arterioles to increasing concentrations of adenosine (ADE) (A) and serotonin (SER) (B) at three acclimation temperatures (1°C, 5°C, and 10°C). Dissimilar lower-case letters indicate significant differences in the response of vessels to the various concentrations of ADE or SER at a given acclimation temperature. Dissimilar capital letters indicate significant differences in the response of vessels at different acclimation temperatures to the same concentration of ADE or SER. To improve clarity of the figure, capital letters are not included when differences were not significant (i.e., AAA). P < 0.05, except where indicated (*), where 0.1 > P ≥ 0.05. Note: a series of sham saline injections did not result in noticeable (i.e., <0.6%) changes in vessel ID. Thus we considered any change in vessel ID greater than the mean change + 2 SD caused by the sham saline injections (∼3%) to be biologically significant.
Low concentrations (10−10 to 10−8 M) of SER caused a mild constriction of the vessels at 1°C and 5°C, whereas higher concentrations caused the vessels to dilate by ∼12% of their resting ID (Fig. 3B). In contrast, coronary arterioles from 10°C acclimated trout exclusively showed a concentration-dependent dilation to SER that reached 30% of resting ID at the highest concentration (10−5 M). This was ∼2.5-fold greater than observed at 1°C or 5°C.
Acetylcholine and bradykinin.
In general, ACh induced a constriction of the trout coronary arterioles (Fig. 4A). However, this effect was concentration and temperature dependent. Although vessels at 10°C showed a very minor dilation (3%) at the lowest ACh concentration used (10−9 M), this effect disappeared as the concentration increased, and this resulted in a small, but significant, constriction of ∼6.5% at higher concentrations. At 1°C and 5°C no vasodilation was observed at low concentrations of ACh. Instead, vessels at 1°C and 5°C showed a very clear concentration-dependent constriction in response to ACh, the extent of this constriction reaching 13.7% and 18.2% at 10−4 M at 1°C and 5°C, respectively. This was a two- to threefold greater constriction (10−6-10−4 M) compared with vessels at 10°C.
Fig. 4.
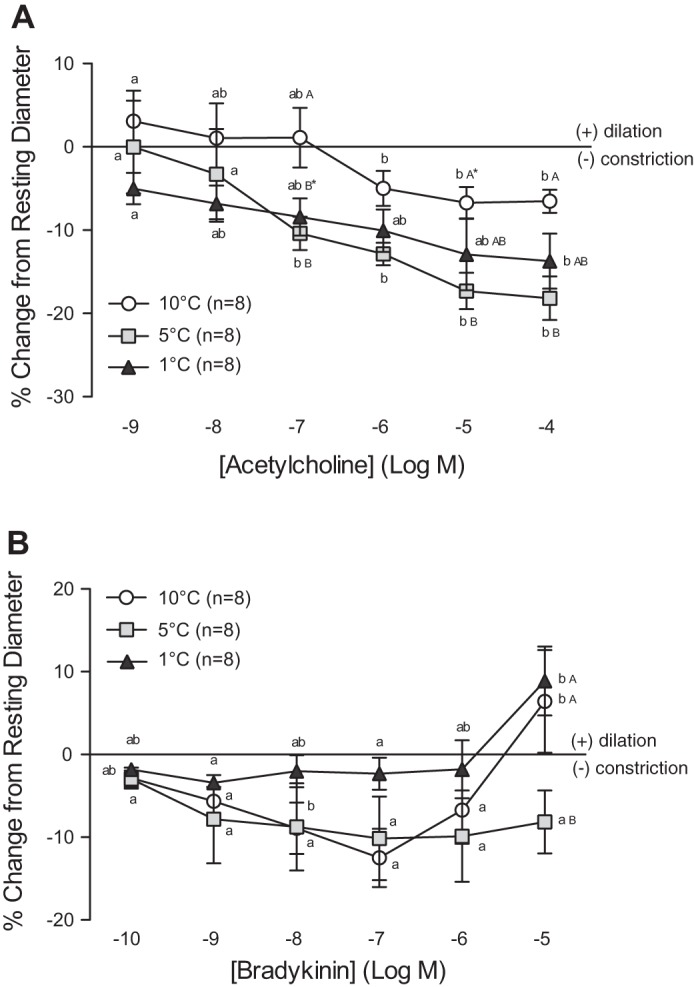
Vasomotor responses of trout coronary arterioles to increasing concentrations of acetylcholine (ACh) (A) and bradykinin (BK) (B) at three acclimation temperatures (1°C, 5°C, and 10°C). Dissimilar lower-case letters indicate significant differences in the response of vessels to the various concentrations of ACh or BK, at a given acclimation temperature. Dissimilar capital letters indicate significant differences in the response of vessels at different acclimation temperatures to the same concentration of ACh or BK. To improve clarity of the figure, capital letters are not included when differences were not significant (i.e., AAA). P < 0.05, except where indicated (*), where 0.1 > P ≥ 0.05. Note: a series of sham saline injections did not result in noticeable (i.e., <0.6%) changes in vessel ID. Thus we considered any change in vessel ID greater than the mean change + 2 SD caused by the sham saline injections (∼3%) to be biologically significant.
The effect of BK on vessel diameter was also dependent on acclimation temperature (Fig. 4B). At 5°C, BK exclusively caused arterioles from trout to constrict (i.e., by 8% from 10−9 to 10−5 M). In contrast, vessels at 10°C showed a biphasic response. At 10°C, BK caused arteriole constriction from 10−10 to 10−7 M (maximum 12.5% from resting ID at 10−7 M) followed by a dilation that reached 6.4% of resting ID at 10−5 M. Vessels from fish acclimated to 1°C showed no change (i.e., only ∼2% constriction) in ID from 10−10 to 10−6 M but a dilation of ∼9% of resting ID at 10−5 M.
Endothelin-1 and sodium nitroprusside.
The sensitivity of the vessels to ET-1 was significantly greater at the lowest temperature in this study (1°C), with vessel constriction being 1.3- to 2-fold higher compared with 5°C vessels and 3.5- to 4-fold higher than measured at 10°C (10−9 and 10−8 M). Nevertheless, the maximum level of constriction achieved (∼80% at 10−7 M) was not different between the groups (Fig. 5A).
Fig. 5.
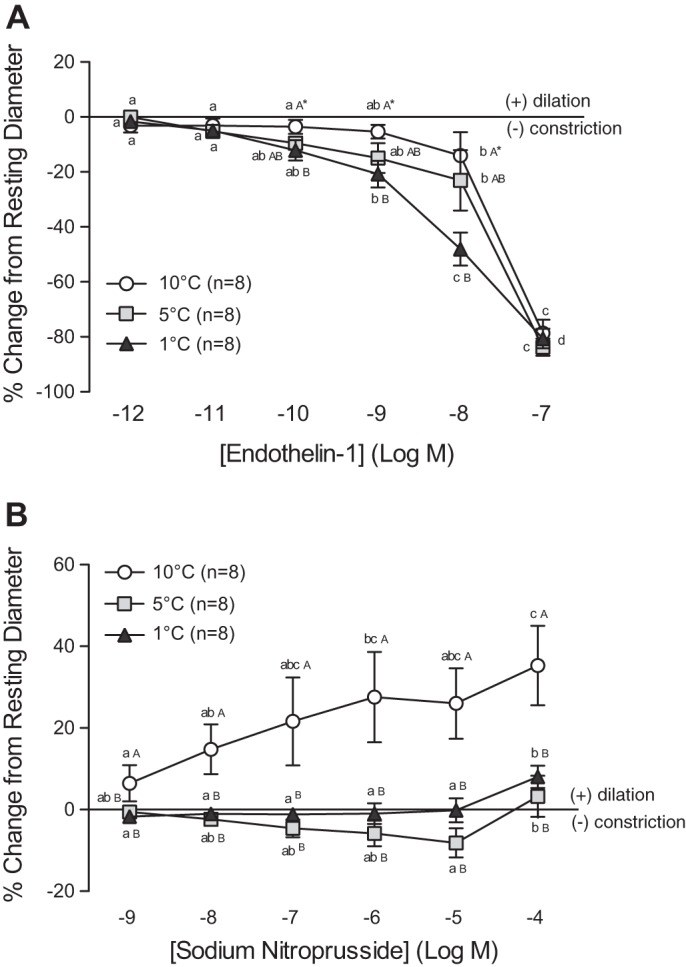
Vasomotor responses of trout coronary arterioles to increasing concentrations of endothelin-1 (ET-1) (A) and sodium nitroprusside (SNP) (B) at three acclimation temperatures (1°C, 5°C, and 10°C). Dissimilar lower-case letters indicate significant differences in the response of vessels to the various concentrations of ET-1 or SNP, at a given acclimation temperature. Dissimilar capital letters indicate significant differences in the response of vessels at different acclimation temperatures to the same concentration of ET-1 or SNP. To improve clarity of the figure, capital letters are not included when differences were not significant (i.e., AAA). P < 0.05, except where indicated (*), where 0.1 > P ≥ 0.05. Note: a series of sham saline injections did not result in noticeable (i.e., <0.6%) changes in vessel ID. Thus we considered any change in vessel ID greater than the mean change + 2 SD caused by the sham saline injections (∼3%) to be biologically significant.
The response of coronary microvessels to SNP was different at all three test temperatures (Fig. 5B). Vessels from trout acclimated to 5°C showed a slight concentration-dependent constriction when exposed to 10−9 to 10−5 M of SNP, followed by a small (3.3%) dilation at 10−4 M. Vessels from 1°C-acclimated trout were largely unresponsive from 10−9 to 10−4 M SNP but showed a clear (8%) vasodilation at 10−4 M. Finally, at 10°C, SNP only caused a strong concentration-dependent vasodilation, with a maximal dilation of 40% of resting ID at the highest concentration used (10−4M). This response was 4-fold and 11-fold greater than observed in 1°C and 5°C vessels, respectively.
DISCUSSION
To our knowledge, with the exception of one study on hagfish (Myxine glutinosa; 33), this is the only study where the response of isolated fish resistance vessels to vasoactive agents has been examined. Here, we demonstrate the feasibility/utility of microvessel techniques for fish research and show that the response of these vessels to several important vasoactive agents is concentration and temperature dependent. Interestingly, these responses often differ considerably from those observed in larger (conduit) vessels (i.e., coronary artery) (26, 92, 93) or in the whole coronary circulation (1, 69, 70).
Important Mediators of Vascular Tone
Catecholamines.
Variable results have been obtained in previous studies that investigated the effects of EPI and NE on the trout (O. mykiss) coronary circulation. Isolated coronary artery rings generally show a concentration-dependent vasodilation to EPI and NE, with 10−3 M of either catecholamine inducing maximum dilation (93). Whereas, a strong constrictor response is seen in the intact perfused coronary circulation (nonworking heart), with coronary vascular resistance (RCor) increasing by 40% with EPI (10−5 M) and more than 100% with NE (10−5 M) (1, 68). In this study, a slight constriction (3–13% from resting ID) was observed when coronary microvessels were exposed to low concentrations of both EPI and NE (≤10−7 M), suggesting the activation of α-adrenoreceptors (ARs). However, higher concentrations of either catecholamine led to a concentration-dependent dilation that reached 32–34% of resting ID at 10−4 M. Although we cannot make any definitive statements regarding the heterogeneity of trout coronary vessel responses to EPI and NE (i.e., 1 vs. 93), in the current study we used true resistance vessels that had a functional endothelium (Costa IASF, Hein TW, Secombes CJ, and Gamperl AK; unpublished observations vs. coronary artery rings with possibly damaged endothelium due to the ring mounting process, 93), and yet, no strong constrictor response was observed with either EPI or NE. It is thus probable that the adrenergic-induced increase in RCor observed in both trout and mammals is caused by a mechanism(s) other than direct α-AR stimulation (49). For example, stimulation of cardiomyocyte α1-ARs leads to the release of angiotensin, which in turn stimulates the release of ET-1 from the vascular endothelium of the coronary arterioles (108), and thus, vasoconstriction (16, 99, 108). ET-1 has a strong constrictor effect on the mammalian coronary circulation (51, 61) and on isolated trout microvessels (see Endothelin-1).
Adenosine.
ADE appears to have both constrictor and dilator effects depending on the vascular bed, vessel size, species, and concentration used (41, 43, 58, 60). In trout, ADE has been shown to constrict isolated coronary artery rings in a concentration-dependent manner (10−8 to 10−3 M; with maximum constriction at 10−3 M) (26, 93), but to cause a biphasic response in the coronary circulation of the nonworking heart (with a 25% increase in Rcor at 10−9 to 10−8 M, but a decrease at 10−7 to 10−5 M; 69). Finally, in the present study, ADE was a powerful dilator of isolated trout coronary arterioles at all concentrations and caused a dilation of 60% from resting ID at 10−4 M.
In both mammals and fish, ADE-induced vasomotor effects are mediated by A1 (vasoconstriction) and A2 (vasodilation) purine receptors (18, 97). Different populations of ADE receptors in conduit vessels (A1) versus the microcirculation (A2) would explain the different responses observed between isolated trout coronary arteries (i.e., conduit vessels; 26, 93) and microvessels (present study), and there is support for such a hypothesis. Preferential action of A2 receptors in smaller vessels has previously been demonstrated in mammals (55). Furthermore, a heterogeneous distribution of ADE receptors has been shown for the porcine coronary circulation (43) and suggested for both mammals (41) and fish (69, 85); the latter based on the biphasic response of the perfused coronary circulation to increasing concentrations of ADE. Nonetheless, ADE-mediated vasoconstriction of the trout coronary artery could potentially reduce qCor to the myocardium during periods of high metabolic stress such as hypoxia or exercise, and thus, the existence of such regulation is highly questionable.
Serotonin.
In this study, SER induced a concentration-dependent dilation of trout coronary microvessels that reached a maximum of 30% at 10−5 M. This result is consistent with Mustafa et al. (70) who showed that SER causes a concentration-dependent decrease in RCor (i.e., dilation) of the isolated nonworking heart of trout (maximum 20% at 10−4 M). A SER-mediated concentration-dependent dilation is also observed in the coronary artery of skate (Raja nasuta) (23), trout, and dogfish (Squalus acanthias; 26). However, Small et al. (93) showed that SER caused dilation of the trout coronary arteries at low concentrations (≤10−6 M) but constriction at higher concentrations (≥10−4 M). In contrast, SER did not affect coronary artery vasoactivity in the mako shark (Isurus oxyrinchus; 24). Again, the heterogeneous responses observed in the above studies may be due to differences in SER receptor distribution between species and/or SERs mechanism(s) of action in the coronary circulation. In mammals, serotonergic receptor subtypes present in the coronary vasculature vary with species (32), age (72, 73, 103), and vessel size (32, 56). Furthermore, SER is able to mediate vasoconstriction and vasodilation simultaneously, through multiple mechanisms (44, 45).
Clearly, more research is needed to accurately characterize the role of SER in the regulation of qCor in fish. For example, SER-induced vasodilation through mechanisms involving an endothelium-derived relaxing factor (EDRF) has been repeatedly suggested for mammalian coronary vessels (45, 62, 63, 83).
Acetylcholine.
In fish, the general response to ACh stimulation has been reported as vasoconstriction. In fact, with the exception of two studies on elasmobranchs (23, 24), all other previous studies on isolated conductance vessels show that ACh induces a concentration-dependent constriction (26, 47, 64, 76, 93).
In trout, ACh induces a strong concentration-dependent constriction (70–95%) of isolated coronary arteries (70–95%; 26, 92, 93), and the majority of nonworking heart preparations show a concentration-dependent increase in RCor (40% change at 10−3 M) with this agonist (70). The biphasic response (dilation followed by constriction) reported in this study is in contrast with these earlier results. However, it is not unique. Mustafa et al. (70) showed that the concentration-response curve to ACh was biphasic in some preparations; with ACh inducing a decrease in RCor (i.e., vasodilation) at low concentrations (≤10−7 M).
The difference in the type (constriction vs. dilation) and magnitude of vasomotor responses to ACh can probably be explained by the balance between direct stimulation of the vascular smooth muscle (VSM; i.e., constriction) and EDRF-mediated dilation (10, 31, 107). For example, the above results suggest that the distribution of cholinergic receptors in the trout coronary vasculature is varied, with a larger influence of VSM cholinergic innervation in large conduit vessels (70–95% constriction in coronary artery; 26, 92, 93) compared with the microvasculature (6.5%; present study). This would be consistent with Farrell and Johansen (26) who showed that atropine (an antagonist of muscarinic receptors) completely blocked the constrictor effect of ACh on trout coronary arteries. Nonetheless, it appears that a small endothelium-dependent contribution to vasoconstriction exists, since endothelium removal only reduced the sensitivity of vessels to low concentrations of ACh (26) and attenuated the constriction of isolated microvessels to a single high concentration (10−4 M) of ACh (data not shown). Finally, the existence of a small endothelium-dependent vasodilation mediated through ACh signaling cannot be disregarded, since vasodilation to low concentrations of ACh was observed both by Mustafa et al. (70) and in the present study.
Bradykinin.
In mammals, BK generally lowers arterial vascular resistance by stimulating a number of secondary mediators (e.g., EDRFs) with the exact mediator varying between species (9). In trout (O. mykiss), BK leads to a multiphasic (vasoconstrictor-vasodilator) effect on dorsal aortic pressure (79). A biphasic response to BK was also seen in isolated trout coronary microvessels, with constriction at low concentrations (10−10 to 10−7 M, max. 12.5%) and dilation at 10−5 M (by 6.4%) (Fig. 4B). In contrast, this has not been observed in isolated coronary artery rings (26, 93), the ventral aorta, or the celiacomesenteric artery (76).
The biphasic effect of BK on trout coronary microvessels may be due to differences in the affinity of the BK receptors present and/or the involvement of secondary mediators (9, 12). For example, Olson et al. (79) suggested that none of the BK-mediated effects on trout vessels are due to direct stimulation of the VSM, and there is evidence that BK-induced vasoconstriction occurs indirectly via secondary mediators (13, 87). With regard to the vasodilation observed after BK stimulation, the involvement of prostaglandins has been suggested for both trout (79) and cod (G. morhua; 90). In contrast, the cyclooxygenase blocker indomethacin did not inhibit the BK-induced relaxation in the small mesenteric arteries of hagfish (33).
Endothelin-1.
In all isolated fish vessel studies to date, ET-1 is one of the most potent vasoconstrictors (78, 88, 96). Consistent with that described for similar-sized mammalian coronary microvessels (105) and isolated trout coronary artery rings (78), the constrictor effect of ET-1 on the trout coronary arterioles was long lasting and sustained. Interestingly, ET-1-mediated vasoconstriction of isolated trout coronary microvessels was only initiated once nanomolar concentrations were reached (∼7% change from resting ID at 10−9 M) suggesting that, similar to mammals, the role of ET-1 in the regulation of vascular resistance is likely that of a paracrine or autocrine mediator versus a circulating hormone (61).
Sodium nitroprusside.
Although the endothelial-NO signaling system is very important in the regulation of vascular tone in mammals (7, 65, 66), its existence in fish has been questioned by a number of authors (47, 50, 76, 82). Some of these authors have suggested that the endothelium-dependent dilation observed in fish vessels is due solely to the action of prostaglandins (50, 76, 82) and that NO does not have a predominant role in regulating vasodilation.
In the present study, the NO donor SNP induced a concentration-dependent dilation of isolated trout coronary microvessels with a maximal dilation of 40% from resting vessel ID at 10−4 M. This supports previous research showing that the VSM of teleost fish contains an NO-dependent signaling cascade that mediates vasodilation. Various NO donors (e.g., nitroglycerine and SNP) induce a concentration-dependent dilation in various vessels in trout and other fish species (47, 64, 76, 80, 85). Furthermore, evidence of a NO signaling system in the trout coronary circulation has been obtained using perfused nonworking hearts, where vascular resistance was decreased and increased by the NO precursor l-arginine and NO synthase (NOS) inhibitors, respectively (69, 70).
Whether or not a source of endothelial NO exists in the trout coronary microvasculature remains to be seen. In the trout coronary vascular bed, Mustafa et al. (70) reported that l-arginine-mediated vasodilation was eliminated by NOS inhibitors, showing that NO or NO-like factor(s) production is possible in the trout coronary vascular bed. Nonetheless, it is also possible that a nonendothelial source of NO exists. In mammals, nitrergic nerves provide a second potential source of NO control of vascular tone (34, 100), and a similar mechanism has been suggested for the eel (Anguila australis). In this species neuronal NOS (nNOS) was detected in the perivascular nerves of the eel aorta and intestinal vein (47). In addition, endothelial NOS (eNOS) is localized in ventricular cardiomyocytes (spongy and compact layers) in several fish species (102), and enzyme-independent NO production from nitrites can be as important, and sometimes exceed, NOS-dependent NO synthesis (67). Thus it is possible that a combination of NO synthetic pathways (NOS-dependent and nonenzymatic) could exist in the trout coronary microcirculation. Future work should focus on establishing the possible sources of NO in the trout coronary microcirculation (eNOS, nNOS, and nonenzymatic sources), as well as further delineating the mechanism(s) of NO-mediated vascular regulation.
Effect of Acclimation Temperature on Vasoactive Agents
Acclimation temperature affected the response of microvessels to most of the vasoactive agents used in this study. However, this response was neither unidirectional nor consistent, and the interpretation of these results is very challenging due to the lack of studies that have examined the effect of temperature on fish vessel physiology.
Catecholamines.
There was no effect of acclimation temperature on the vasoactive response of coronary microvessels to NE. However, acclimation to 1°C decreased the dilation observed with EPI at 10−5 and 10−4 M by ∼90 and 60%, respectively. These results are in contrast to Farrell (19) who showed that adrenergic-mediated vasoconstriction of the trout coronary circulation (using a perfused heart setup) was significantly greater in hearts of trout acclimated to 5°C versus 15°C, and to Stecyk et al. (94) who suggested that the adrenergic regulation of systemic vasomotor tone is more important at low temperatures in the turtle (Trachemys scripta) due to an increase in the systemic α-adrenergic tone. Furthermore, the present findings are at odds with research showing that cold acclimation increases myocardial β-ARs density (52) as well as the sensitivity of L-type Ca2+ channels (91) in trout.
Adenosine.
The vasodilator response of trout coronary microvessels to ADE was 1.2- to 2.5-fold higher in vessels from fish acclimated to 10°C (10−7 to 10−4 M) compared with arterioles from fish acclimated to either 1°C or 5°C. This suggests that the sensitivity of the coronary circulation to ADE decreases with cold acclimation. Aho and Vornanen (2) report that the effects of ADE on cardiac contractility and electrical activity are also weaker in cold-acclimated fish (4°C vs. 17°C). Given the apparent relationship between myocardial metabolism and regulation of vascular tone by adenosine (see Important Mediators of Vascular Tone, Adenosine), it is not surprising that the action of this metabolite is reduced at cold temperatures. Higher temperatures are generally associated with increases in metabolic rate and tissue (e.g., myocardial) metabolic demand (35, 75), and thus, the regulation of qCor (e.g., via ADE release) would need to match these energetic demands so that appropriate levels of cardiac performance are maintained.
Serotonin and acetylcholine.
Acclimation to 1°C and 5°C affected the balance between dilation and constriction produced by both of these agents in favor of the latter. Acclimation to these temperatures blunted the dilation of trout coronary arterioles at high concentrations of SER (≥10−7 M) and changed the response of vessels at lower concentrations (≤10−8 M) to a mild constriction (Fig. 3B). This finding is interesting given the significant SER-induced branchial vasoconstriction observed in some Antarctic fish species (71, 84). In fact, cold adaptation may explain the high sensitivity to SER observed in these species. Given that SER is able to simultaneously mediate vasoconstriction and vasodilation (44, 45, Fig. 3B), it is possible that an increase in SER-induced vasoconstriction (e.g., due to altered receptor affinity) occurs at these lower acclimation temperatures and that this diminishes the dilation produced by SER. Similarly, acclimation to 5°C and 1°C eliminated the slight vasodilation observed at 10−9 M ACh and significantly enhanced (by 2- to 3-fold) the constriction seen at all other concentrations. It is well known that the relative importance of cholinergic tone on the heart can change dramatically between species acclimated to different temperatures (89), and that cholinergic mechanisms of cardiac regulation in trout appear to be more important at lower acclimation temperatures (35, 106).
Bradykinin, endothelin-1, and sodium nitroprusside.
Acclimation temperature had the most variable effect on the vasoactivity of trout coronary microvessels to BK. The biphasic response observed at 10°C changed to a mild constriction (by about 8%) over the range of concentrations tested at 5°C and to vessels showing no response to BK (at 1°C) except for a small dilation (∼6.5%) at the highest concentration (10−5 M).
Although the maximum level of vessel constriction observed with ET-1 (∼80% from resting ID at 10−7 M) was independent of acclimation temperature, vessels from fish acclimated to 1°C were more sensitive to ET-1 at 10−10 and 10−8 M compared with vessels from animals acclimated to 10°C (Fig. 5A). Increased potency of ET-1 with cold exposure has also been shown in mammals. Low temperature increased the concentration-dependent constriction of rabbit ear vessels by ET-1 (81).
There was a clear temperature effect on the response of trout coronary arterioles to SNP. Low acclimation temperatures (1°C and 5°C) almost completely abolished the concentration-dependent dilation observed in vessels at 10°C. Although data on the effects of temperature on NO-mediated vascular regulation in vertebrates are scarce, and variable, it is clear that temperature can potentially influence NO vasoactivity in a variety of taxa. For example, attenuation of NO-mediated vasodilation of the splanchnic vasculature has been reported in hyperthermic rats (39), whereas heat stress has been shown to increase eNOS protein content in rat aorta (40), and cold exposure has been shown to decrease eNOS expression in the pulmonary arterioles of broilers (98).
Perspectives and Significance
In this research, we show that the dose-dependent responses of isolated trout coronary microvessels (67 to 146 μm ID) to important vasoactive agents can be dramatically affected by temperature, with cold temperatures resulting in a range of effects, from no alteration in vasoactivity to a complete elimination of vasomotor responses. These results contribute significantly to our understanding of how low temperatures affect fish cardiovascular physiology and suggest that the predominant factors that control qCor and vascular resistance, in general, may change with temperature. They also raise the possibility that dysregulation of vasomotor control contributes to the lower and upper thermal tolerance of fishes. For example, acclimation to low temperatures almost completely abolished the concentration-dependent dilation achieved with the NO donor SNP, and this gasotransmitter plays a critical role in temperature-dependent cardiovascular function in fishes (3, 46). In addition, limitations in cardiovascular function are currently considered to be the primary determinant of the upper thermal tolerance of fishes (29, 35), and heat stress in both fish and mammals is associated with large changes in blood pressure and vascular resistance (38, 57). It has been suggested that these hemodynamic effects are related to the release of the cytokine interleukin-1β at high temperatures (6, 57, Costa IASF, Hein TW, Secombes CJ, and Gamperl AK; unpublished observations). However, these responses could also be related to the inability of the vasculature to appropriately compensate for the vascular effects of this immune molecule. Clearly, future studies of this type will be extremely important in further understanding how vascular resistance is regulated in fishes under varied environmental conditions and the contribution of hormonal, neural, and locally mediated control mechanisms to blood flow regulation in this taxa.
GRANTS
This research was supported by an National Sciences and Engineering Research Council Discovery and Accelerator Supplement grants to A. K. Gamperl, a National Institutes of Health Grant (EY018420) to T. W. Hein, and a doctoral fellowship from Fundação para a Ciência e a Tecnologia (FCT, Portugal) to I. A. S. F Costa.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: I.A.S.F.C., T.W.H., and A.K.G. conception and design of research; I.A.S.F.C. performed experiments; I.A.S.F.C. analyzed data; I.A.S.F.C. and A.K.G. interpreted results of experiments; I.A.S.F.C. prepared figures; I.A.S.F.C. drafted manuscript; I.A.S.F.C., T.W.H., and A.K.G. edited and revised manuscript; I.A.S.F.C., T.W.H., and A.K.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Wenjuan Xu and Xin Xu (Hein Lab) for excellent instruction in microvessel techniques.
REFERENCES
- 1.Agnisola C, Mustafa T, Hansen JK. Autoregulatory index, adrenergic responses, and interaction between adrenoreceptors and prostacyclin in the coronary system of rainbow trout. J Exp Zool 275: 239–248, 1996. [Google Scholar]
- 2.Aho E, Vornanen M. Effects of adenosine on the contractility of normoxic rainbow trout heart. J Comp Physiol B 172: 217–225, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Amelio D, Garofalo F, Capria C, Tota B, Imbrogno S. Effects of temperature on the nitric oxide-dependent modulation od the Frank-Starling mechanism: the fish heart as a case study. Comp Biochem Physiol 164A: 356–362, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Axelsson M. The coronary circulation: a fish perspective. Braz J Med Biol Res 28: 1167–1177, 1995. [PubMed] [Google Scholar]
- 5.Axelsson M, Farrell AP. Coronary blood flow in vivo in the coho salmon (Onchorhynchus kisutch). Am J Physiol Regul Integr Comp Physiol 264: R963–R971, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Bataillard A, Sassard J. Cardiovascular effects of human recombinant interleukin-1β in conscious rats. Am J Physiol Regul Integr Comp Physiol 266: R1148–R1153, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Bauer V, Stníková R. Nitric oxide–the endothelium-derived relaxing factor and its role in endothelial functions. Gen Physiol Biophys 29: 319–340, 2010. [PubMed] [Google Scholar]
- 8.Berne RM, Levy MN. Cardiovascular Physiology (7th ed) Philadelphia, PA: Mosby-Year Book, 1997, p. 171–194. [Google Scholar]
- 9.Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins- kallikreins, kininogens, and kininases. Pharmacol Rev 44: 1–80, 1992. [PubMed] [Google Scholar]
- 10.Bolz SS, de Wit C, Pohl U. Endothelium-derived hyperpolarizing factor but not NO reduces smooth muscle Ca2+ during acetylcholine-induced dilation of microvessels. Br J Pharmacol 128: 124–134, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol Heart Circ Physiol 251: H779–H788, 1986. [DOI] [PubMed] [Google Scholar]
- 12.Conlon JM. Bradykinin and its receptors in non-mammalian vertebrates. Regul Pept 79: 71–81, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Dasiewicz PJ, Michael CJ, Anderson WG. Cardiovascular and vasoconstrictive actions of skate bradykinin in the little skate, Leucoraja erinacea (Elasmobranchii). Gen Comp Endocrinol 174: 89–96, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Davie PS, Farrell AP. The coronary and luminal circulations of the myocardium of fishes. Can J Zool 69: 1993–2001, 1991. [Google Scholar]
- 15.Daxboeck C. Effect of coronary ablation on exercise performance in Salmo gairdneri. Can J Physiol 60: 375–381, 1982. [Google Scholar]
- 16.DeFily DV, Nishikawa Y, Chilian WM. Endothelin antagonists block α1-adrenergic constriction of coronary arterioles. Am J Physiol Heart Circ Physiol 276: H1028–H1034, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Duling BR, Gore RW, Dacey RG Jr, Damon DN. Methods for isolation canulation and in vitro study of single microvessels. Am J Physiol Heart Circ Physiol 241: H108–H116, 1981. [DOI] [PubMed] [Google Scholar]
- 18.Evans DH. Evidence for the presence of A1 and A2 adenosine receptors in the ventral aorta of the dogfish shark, Squalus acanthias. J Comp Physiol B 162: 179–183, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Farrell AP. Coronary flow in a perfused rainbow trout heart. J Exp Biol 129: 107–123, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Farrell AP. Features heightening cardiovascular performance in fishes with special reference to tunas. Comp Biochem Physiol A 113: 61-7, 1996. [Google Scholar]
- 21.Farrell AP. The coronary circulation. In: Encyclopedia of Fish Physiology: From Genome to Environment, edited by Farrell AP. San Diego, CA: Academic, 2011. [Google Scholar]
- 22.Farrell AP, Steffensen JF. Coronary ligation reduces maximum sustained swimming speed in chinook salmon, Oncorhynchus tshawytscha. Comp Biochem Physiol A 87: 35–37, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Farrell AP, Davie PS. Coronary vascular reactivity in the skate, Raja nasuta. Comp Biochem Physiol C 99: 555–560, 1991. [Google Scholar]
- 24.Farrell AP, Davie PS. Coronary artery reactivity in the mako shark, Isurus oxyrinchus. Can J Zool 69: 375–379, 1991. [Google Scholar]
- 25.Farrell AP, Jones DR. The heart. In: Fish Physiology, edited by Hoar WS, Randall DJ, Farrell AP. San Diego, CA: Academic, vol. XIIA, 1992. [Google Scholar]
- 26.Farrell AP, Johansen JA. Vasoactivity of the coronary artery of rainbow trout, steelhead trout, and dogfish: lack of support for non-prostanoid endothelium-derived relaxation factors. Can J Zool 73: 1899–1911, 1995. [Google Scholar]
- 27.Farrell AP, Johansen JA, Steffensen SF, Moeyes CD, West TG, Suarez RK. Effects of exercise training and coronary ablation on swimming performance, heart size, and cardiac enzymes in rainbow trout, Oncorhynchus mykiss. Can J Zool 68: 1174–1179, 1990. [Google Scholar]
- 28.Farrell AP, Davie PS, Franklin CE, Brill RW. Cardiac physiology in tunas. I. In vitro perfused heart preparations from yellowfin and skipjack tunas. Can J Zool 70: 1200–1210, 1992. [Google Scholar]
- 29.Farrell AP, Eliason EJ, Sandblom E, Clark TD. Fish cardiorespiratory physiology in an era of climate change. Can J Zool 87: 835–851, 2009. [Google Scholar]
- 30.Feigl EO. Coronary physiology. Physiol Rev 63: 1–205, 1983. [DOI] [PubMed] [Google Scholar]
- 31.Feigl EO. Neural control of coronary blood flow. J Vasc Res 35: 85–92, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Félétou M, Dellazuana O, Duhault J. Serotoninergic receptor subtype in coronary artery smooth muscle from young and atherosclerotic rabbit. J Pharmacol Exp Ther 268: 124–132, 1994. [PubMed] [Google Scholar]
- 33.Feng J, Yano K, Monahan-Earley R. Vascular bed-specific endothelium-dependent vasomomotor relaxation in the hagfish, Myxine glutinosa. Am J Physiol Regul Integr Comp Physiol 293: R894–R900, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 33: 829–837, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gamperl AK. Integrated responses of the circulatory system to temperature. In: Encyclopedia of Fish Physiology: From Genome to Environment, edited by Farrell AP. San Diego, CA: Academic, 2011. [Google Scholar]
- 36.Gamperl AK, Pinder AW, Boutilier RG. Influence of hypoxia and adrenaline administration on coronary blood flow and cardiac performance in trout (Oncorhynchus mykiss). J Exp Biol 193: 209–232, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Gamperl AK, Axelsson M, Farrell AP. Effects of swimming and environmental hypoxia on coronary blood flow in rainbow trout. Am J Physiol Regul Integr Comp Physiol 269: R1258–R1266, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Gamperl AK, Swafford BL, Rodnick KJ. Elevated temperature, per se, does not limit the ability of rainbow trout to increase ventricular stroke volume. J Therm Biol 36: 7–14, 2011. [Google Scholar]
- 39.Haddad W, Horowitz M. Heat acclimation alters nitric oxide response in the splanchnic circulation. J Therm Biol 24: 403–408, 1999. [Google Scholar]
- 40.Harris MB, Blackstone MA, Ju H, Venema VJ, Venema RC. Heat-induced increases in endothelial NO synthase expression and activity and endothelial NO release. Am J Physiol Heart Circ Physiol 285: H333–H340, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Harrison GJ, Harden FA, Jordan LR, Varela JI, Willis RJ. A method to evaluate the response of the coronary circulation of perfused rat heart to adenosine. Can J Physiol Pharmacol 74: 145–149, 1996. [PubMed] [Google Scholar]
- 42.Hein TW, Kuo L. LDLs impair vasomotor function of the coronary microcirculation. role of superoxide anions. Circ Res 83: 404–414, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Hein TW, Wang W, Zoghi B, Muthuchamy M, Kuo L. Functional and molecular characterization of receptor subtypes mediating coronary microvascular dilation to adenosine. J Mol Cell Cardiol 33: 271–282, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Houston DS, Vanhoutte PM. Serotonin and the vascular system. role in health and disease and implications for therapy. Drugs 31: 149–163, 1986. [DOI] [PubMed] [Google Scholar]
- 45.Houston DS, Vanhoutte PM. Comparison of serotonergic receptor subtypes on the smooth muscle and endothelium of the canine coronary artery. J Pharmacol Exp Ther 244: 1–10, 1988. [PubMed] [Google Scholar]
- 46.Imbrogno S, De luri L, Mazza R, Tota B. Nitric oxide modulates cardiac performance in the heart of Anguilla Anguilla. J Exp Biol 204: 1719–1727, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Jennings BL, Broughton BRS, Donald JA. Nitric oxide recontrol of the dorsal aorta and the intestinal vein of the Australina short-finned eel Anguilla australis. J Exp Biol 207: 1295–1303, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Jones CJH, Kuo L, Davis MJ, Chilian WM. Regulation of coronary blood flow: coordination of heterogenous control mechanisms in vascular microdomains. Cardiol Res 29: 585–596, 1995. [PubMed] [Google Scholar]
- 49.Jones CJH, Kuo L, Davis MJ, Chilian WM. α-Adrenergic responses of isolated canine coronary microvessels. Basic Res Cardiol 90: 61–69, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Kågström J, Holmgren S. VIP-induced relaxation of small arteries of the rainbow trout, Oncorhynchus mykiss, involves prostaglandin synthesis but not nitric oxide. J Auton Nerv Syst 63: 68–76, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Karwatowska-Prokopczuk E, Wennmalm Å. Endothelium-derived constricting factor(s): the last novelty-endothelin. Clin Physiol 10: 113–121, 1990. [DOI] [PubMed] [Google Scholar]
- 52.Keen JE, Vianzon DM, Farrell AP, Tibbits GF. Thermal acclimation alters both adrenergic sensitivity and adrenoreceptor density in cardiac tissue of rainbow trout. J Exp Biol 181: 27–47, 1993. [Google Scholar]
- 53.Kuo L, Davis MJ, Chilian WM. Myogenic activity in isolated subepicardial and subendocardial coronary arterioles. Am J Physiol Heart Circ Physiol 255: H1558–H1562, 1988. [DOI] [PubMed] [Google Scholar]
- 54.Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol 259: H1063–H1070, 1990. [DOI] [PubMed] [Google Scholar]
- 55.Kuo L, Davis MJ, Chilian WM. Longitudinal gradients for endothelium-dependent and -independent vascular responses in the coronary microcirculation. Circulation 92: 518–525, 1995. [DOI] [PubMed] [Google Scholar]
- 56.Lamping KG, Kanatsuka H, Eastham CL, Chilian WM, Marcus ML. Nonuniform vasomotor responses of the coronary microcirculation to serotonin and vasopressin. Circ Res 65: 343–351, 1989. [DOI] [PubMed] [Google Scholar]
- 57.Lin MT, Liu HH, Yang YL. Involvement of interleukin-1 receptor mechanisms in development of arterial hypotension in rat heatstroke. Am J Physiol Heart Circ Physiol 273: H2072–H2077, 1997. [DOI] [PubMed] [Google Scholar]
- 58.Lippton HL, Hao Q, Hauth T, Hyman A. Mechanisms of signal transduction for adenosine and ATP in pulmonary vascular bed. Am J Physiol Heart Circ Physiol 262: H926–H929, 1992. [DOI] [PubMed] [Google Scholar]
- 59.Lurman GJ, Petersen LH, Gamperl AK. In situ cardiac performance of Atlantic cod (Gadus morhua) at cold temperatures: long-term acclimation, acute thermal challenge and the role of adrenaline. J Exp Biol 215: 4006–4014, 2012. [DOI] [PubMed] [Google Scholar]
- 60.Makujina SR, Olanrewaju HA, Mustafa SJ. Evidence against KATP channel involvement in adenosine receptor-mediated dilation of epicardial vessels. Am J Physiol Heart Circ Physiol 267: H716–H724, 1994. [DOI] [PubMed] [Google Scholar]
- 61.Masaki T. Historical review: endothelin. Trends Pharmacol Sci 25: 219–224, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Métais C, Li J, Li J, Simons M, Sellke FW. Serotonin-induced coronary contraction increases after blood cardioplegia-reperfusion: role of COX-2 expression. Circulation 100: II328–II334, 1999. [DOI] [PubMed] [Google Scholar]
- 63.Métais C, Bianchi C, Li J, Li J, Simons M, Sellke FW. Serotonin-induced human coronary microvascular contraction during acute myocardial ischemia is blocked by COX-2 inhibition. Basic Res Cardiol 96: 59–67, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Miller VM, Vanhoutte PM. Prostaglandins but not nitric oxide are endothelium-derived relaxing factors in the trout aorta. Acta Pharmacol Sin 21: 871–876, 2000. [PubMed] [Google Scholar]
- 65.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol 147: S193–S201, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moncada S, Palmer RMJ, Higgs EA. Pathophysiology, pharmacology, nitric oxide: physiology. Pharmacol Rev 43: 109–142, 1991. [PubMed] [Google Scholar]
- 67.Moroz LL, Khon AB. On the comparative biology of nitric oxide (NO) synthetic pathways: parallel evolution of NO mediated signaling. In: Nitric Oxide, edited by Tota B, Trimmer B. Oxford, UK: Elsevier, 2007. [Google Scholar]
- 68.Mustafa T, Agnisola C. Vasoactivity of prostanoids in the trout (Oncorhynchus mykiss) coronary system modification by noradrenaline. Fish Physiol Biochem 13: 249–261, 1994. [DOI] [PubMed] [Google Scholar]
- 69.Mustafa T, Agnisola C. Vasoactivity of adenosine in the trout (Oncorhynchus mykiss) coronary system: involvement of nitric oxide and interaction with noradrenaline. J Exp Biol 201: 3075–3083, 1998. [DOI] [PubMed] [Google Scholar]
- 70.Mustafa T, Agnisola C, Hansen JK. Evidence for NO-dependent vasodilation in the trout (Oncorhynchus mykiss) coronary system. J Comp Physiol B 167: 98–104, 1997. [Google Scholar]
- 71.Nilsson S, Sundin L. Gill blood flow control. Comp Biochem Physiol A 119: 137–147, 1998. [DOI] [PubMed] [Google Scholar]
- 72.Nyborg NCB. Aging is associated with increased 5-HT-2-receptor affinity and decreased receptor reserve in rat isolated coronary arteries. Br J Pharmacol 102: 282–286, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nyborg NCB, Mikkelsen EO. Serotonin response increases with age in rat coronary resistance arteries. Cardio Res 22: 131–137, 1988. [DOI] [PubMed] [Google Scholar]
- 74.Olson KR. Vascular actions of hydrogen sulfide in nonmammaliam vertebrates. Antioxid Redox Signaling 7: 804–812, 2005. [DOI] [PubMed] [Google Scholar]
- 75.Olson KR. Physiology of resistance vessels. In: Encyclopedia of Fish Physiology: From Genome to Environment, edited by Farrell AP. San Diego, CA: Academic, 2011. [Google Scholar]
- 76.Olson KR, Villa J. Evidence against nonprostanoid endothelium-derived relaxing factor(s) is trout vessels. Am J Physiol Regul Integr Comp Physiol 260: R925–R933, 1991. [DOI] [PubMed] [Google Scholar]
- 77.Olson KR, Farrell AP. The Cardiovascular System. In: The Physiology of Fishes (3rd ed), edited by Evans DH, Claiborne JB. Boca Raton, FL: Taylor and Francis Group, 2006. [Google Scholar]
- 78.Olson KR, Duff DW, Farrell AP, Keen J, Kellogg MD, Kullman D, Villa J. Cardiovascular effects of endothelin in trout. Am J Physiol Heart Circ Physiol 260: H1214–H1223, 1991. [DOI] [PubMed] [Google Scholar]
- 79.Olson KR, Conklin DJ, Weaver L Jr, Duff DW, Herman CA, Wang X, Conlon JM. Cardiovascular effects of homologous bradykinin in rainbow trout. Am J Physiol Regul Integr Comp Physiol 272: R1112–R1120, 1997. [DOI] [PubMed] [Google Scholar]
- 80.Olson KR, Conklin DJ, Farrell AP, Keen JE, Takei Y, Weaver L Jr, Smith MP, Zhang Y. Effects of natriuretic peptides and nitroprusside on venous function in trout. Am J Physiol Regul Integr Comp Physiol 273: R527–R539, 1997. [DOI] [PubMed] [Google Scholar]
- 81.Padilla J, García-Villalón AL, Monge L, García JL, Fernández N, Gómez B, Diéguez G. Peptidergic modulation of the sympathetic contraction in the rabbit ear artery: effects of temperature. Br J Pharmacol 121: 21–28, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park KH, Kim KH, Choi MS, Choi SH, Yoon JM, Kim YG. Cyclooxygenase-derived products, rather than nitric oxide, are endothelium-derived relaxing factor(s) in the ventral aorta of carp (Cyprinus carpio). Comp Biochem Physiol A 127: 89–98, 2000. [DOI] [PubMed] [Google Scholar]
- 83.Pearson PJ, Lin PJ, Schaff HV, Vanhoutte PM. Augmented endothelium-dependent constriction to hypoxia early and late following reperfusion of the canine coronary artery. Clin Exp Pharmacol Physiol 23: 634–641, 1996. [DOI] [PubMed] [Google Scholar]
- 84.Pellegrino D, Acierno R, Tota B. Control of cardiovascular function in the icefish Chionodraco hamatus: involvement of serotonin and nitric oxide. Comp Biochem Physiol A 134: 471–480, 2003. [DOI] [PubMed] [Google Scholar]
- 85.Pellegrino D, Tota B, Randall DJ. Adenosine nitric oxide crosstalk in the branchial circulation of Squalus acanthias and Anguilla anguilla. Comp Biochem and Physiol A 142: 198–204, 2005. [DOI] [PubMed] [Google Scholar]
- 86.Perry SF, Reid SG. Cardiorespiratory adjustments during hypercarbia in rainbow trout Oncorhynchus mykiss are initiated by external CO2 receptors on the first gill arch. J Exp Biol 205: 3357–3365, 2002. [DOI] [PubMed] [Google Scholar]
- 87.Platzack B, Conlon JM. Purification, structural characterization, and cardiovascular activity of cod bradykinins. Am J Physiol Regul Integr Comp Physiol 272: R710–R717, 1997. [DOI] [PubMed] [Google Scholar]
- 88.Poder TC, Silberberg SD, Rampe D. Contraction of reptile, amphibian and fish blood vessels by endothelin-1. Can J Physiol Pharmacol 69: 215–217, 1991. [DOI] [PubMed] [Google Scholar]
- 89.Sandblom E, Axelsson M. Autonomic control of circulation in fish: a comparative view. Auton Neurosci 165: 127–139, 2011. [DOI] [PubMed] [Google Scholar]
- 90.Shahbazi F, Conlon JM, Holmgrena S, Jensen J. Effects of cod bradykinin and its analogs on vascular and intestinal smooth muscle of the Atlantic cod, Gadus morhua. Peptides 22: 1023–1029, 2001. [DOI] [PubMed] [Google Scholar]
- 91.Shiels HA, Vornanen M, Farrell AP. Acute Temperature change modulates the response of ICa to adrenergic stimulation in fish cardiomyocytes. Physiol Biochem Zool 76: 816–824, 2003. [DOI] [PubMed] [Google Scholar]
- 92.Small SA, Farrell AP. Vascular reactivity of the coronary artery in steelhead trout (Oncorhynchus mykiss). Biochem Physiol 97C: 59–63, 1990. [DOI] [PubMed] [Google Scholar]
- 93.Small SA, MacDonald C, Farrell AP. Vascular reactivity of the coronary artery in rainbow trout (Oncorhynchus mykiss). Am J Physiol Regul Integr Comp Physiol 258: R1402–R1410, 1990. [DOI] [PubMed] [Google Scholar]
- 94.Stecyk JAW, Overgaard J, Farrell AP, Wang T. α-Adrenergic regulation of systemic peripheral resistance and blood flow distribution in the turtle Trachemys scripta during anoxic submergence at 5°C and 21°C. J Exp Biol 207: 269–283, 2004. [DOI] [PubMed] [Google Scholar]
- 95.Steffensen JF, Farrell AP. Swimming performance, venous oxygen tension and cardiac performance of coronary-ligated rainbow trout, Oncorhynchus mykiss, exposed to progressive hypoxia. Comp Biochem Physiol A 119: 585–592, 1998. [DOI] [PubMed] [Google Scholar]
- 96.Sverdrup A, Krüger PG, Helle KB. Role of the endothelium in regulation of vascular functions in two teleosts. Acta Physiol Scand 152: 219–233, 1994. [DOI] [PubMed] [Google Scholar]
- 97.Tabrizchi R, Bedi S. Pharmacology of adenosine receptors in the vasculature. Pharmacol Ther 91: 133–147, 2001. [DOI] [PubMed] [Google Scholar]
- 98.Tan X, Sun WD, Li JC, Pan JQ, Liu YJ, Wang JY, Wang XL. l-Arginine prevents reduced expression of endothelial nitric oxide synthase (NOS) in pulmonary arterioles of broilers exposed to cool temperatures. Vet J 173: 151–157, 2007. [DOI] [PubMed] [Google Scholar]
- 99.Tiefenbacher CP, DeFily DV, Chilian WM. Requisite role of cardiac myocytes in coronary alpha1-adrenergic constriction. Circulation 98: 9–12, 1998. [DOI] [PubMed] [Google Scholar]
- 100.Toda N. Nitrergic (nitroxidergic) innervation in the blood vessel. In: Nitric Oxide and the Peripheral Nervous System, edited by Toda N, Moncada S, Furchgott R, Higgs EA, London, UK: Portland Pres, 2000. [Google Scholar]
- 101.Tota B. Vascular and metabolic zonation in the ventricular myocardium of mammals and fishes. Comp Biochem Physiol A 76: 423–437, 1983. [DOI] [PubMed] [Google Scholar]
- 102.Tota B, Imbrogno S, Mazza R, Gattuso A. NOS distribution and NO control of cardiac performance in fish and amphibian hearts. In: Nitric Oxide, edited by Tota B, Trimmer B. Oxford, UK: Elsevier, 2007. [Google Scholar]
- 103.Tschudi MR, Luscher TF. Age and hypertension differently affect coronary contractions to endothelin-1, serotonin, and angiotensins. Circulation 91: 2415–2422, 1995. [DOI] [PubMed] [Google Scholar]
- 104.Tsukada T, Rubio R, Berne RM. Pharmacologial differentiation between large and small coronary vessels. Arch Int Pharmacodyn Ther 270: 255–267, 1984. [PubMed] [Google Scholar]
- 105.Wang Y, Kanatsuka H, Akai K, Sugimura A, Kumagai T, Komaru T, Sato K, Shirato K. Effects of low doses of endothelin-1 on basal vascular tone and autoregulatory vasodilation in canine coronary microcirculation in vivo. Jpn Circ J 63: 617–623, 1999. [DOI] [PubMed] [Google Scholar]
- 106.Wood CM, Pieprzak P, Trott N. The influence of temperature and anaemia on the adrenergic and cholinergic mechanisms controlling heart rate in rainbow trout. Can J Zool 57: 2440–2447, 1979. [Google Scholar]
- 107.Woodman OL, Wongsawatkul O, Sobey CG. Contribution of nitric oxide, cyclic GMP and K+ channels to acetylcholine-induced dilatation of rat conduit and resistance arteries. Clin Exp Pharmacol Physiol 27: 34–40, 2000. [DOI] [PubMed] [Google Scholar]
- 108.Yamaguchi O, Kaneshiro T, Saitoh S, Ishibashi T, Maruyama Y, Takeishi Y. Regulation of coronary vascular tone via redox modulation in the α1-adrenergic-angiotensin-endothelin axis of the myocardium. Am J Physiol Heart Circ Physiol 296: H226–H232, 2009. [DOI] [PubMed] [Google Scholar]