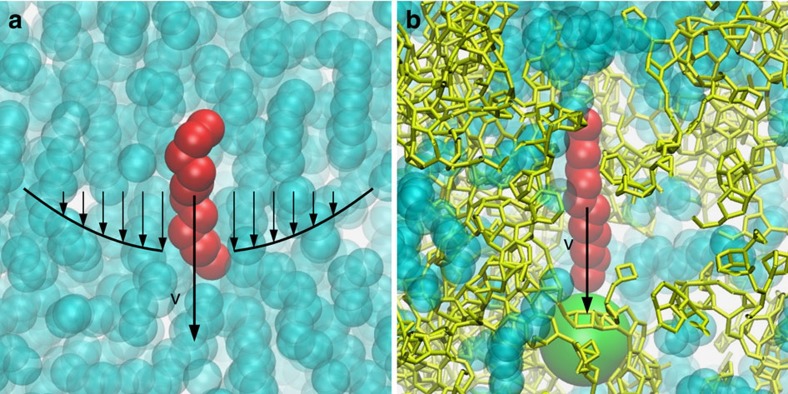
Figure 5. Diffusion mechanisms for bulk alkanes and alkanes confined in the porous carbon matrix.
Left: In bulk, molecular diffusion is well described by the hydrodynamic Stokes–Einstein relation Ds=μkBT=kB/(4πηR0) (with slip boundary conditions). The effective particle diameter 2R0 is consistent with 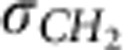 —independently of the alkane length—because the diffusive motion is mostly in longitudinal direction. Right: In contrast, in the nanopores, movement of alkane molecules is dominated by friction on the carbon matrix, corrected for the free volume accessible to the molecule (green sphere). The total friction force is a sum of the forces between the individual monomers with the pore wall—therefore scaling linearly with the alkane length. v stands for the molecule velocity.
—independently of the alkane length—because the diffusive motion is mostly in longitudinal direction. Right: In contrast, in the nanopores, movement of alkane molecules is dominated by friction on the carbon matrix, corrected for the free volume accessible to the molecule (green sphere). The total friction force is a sum of the forces between the individual monomers with the pore wall—therefore scaling linearly with the alkane length. v stands for the molecule velocity.