Table 5. Syntheses of α-alkyloxyl-α,α-diaryl imine.
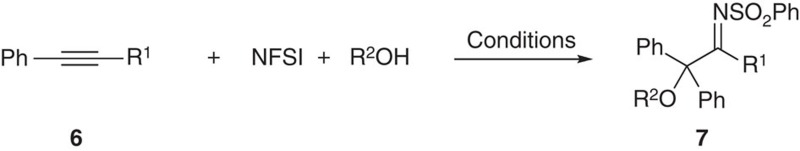 | |||||
|---|---|---|---|---|---|
| Entry | R1 | R2OH | Temperature (oC) | Product | Yield (%) |
| 1 | n-C3H7 | i-PrOH | 0 | 7a | 71 |
| 2 | Me | i-PrOH | 10 | 7b | 68 |
| 3 | n-C4H9 | i-PrOH | 0 | 7c | 63 |
| 4 | n-C3H7 | CH3OH | 10 | 7d | 52 |
| 5 | n-C3H7 | EtOH | 10 | 7e | 62 |
| 6 | n-C3H7 | Butan-1-ol | 10 | 7f | 58 |
| 7 | n-C3H7 | Butan-2-ol | 0 | 7g | 65 |
| 8 | n-C3H7 | Cyclohexanol | 10 | 7h | 70 |
| 9 | n-C3H7 | Prop-2-yn-1-ol | 25 | 7i | 54 |
| 10 | n-C3H7 | (E)-but-2-en-1-ol | 25 | 7j | 46 |
| 11* | Ph | CH3OH | 90 | 7k | 66 |
| 12* | 4-NO2C6H4 | CH3OH | 90 | 7l | 45 |
| 13* | 4-acetyl C6H4 | CH3OH | 90 | 7m:7m′=1.5:1 | 41† |
| 14* | 4-tBuC6H4 | CH3OH | 90 | 7n:7n′=1:2 | 54† |
Reactions condition: 6 (0.5 mmol), NFSI (2 equiv., 1.0 mmol), R2OH (3 equiv., 1.5 mmol), Cu(acac)2 (5 mol %) in CH3CN (2 ml) under N2 atmosphere for 48 h. Isolated yields.
*Reactions condition: 6 (0.5 mmol), NFSI (2 equiv., 1.0 mmol), CH3OH (3 equiv., 1.5 mmol), CuCN (5 mol %) in CH2Cl2 (2 ml) at 90 °C under N2 atmosphere for 48 h. Isolated yields.
†Mixture of two isomers. The ratio was determined by 1H NMR analysis.
