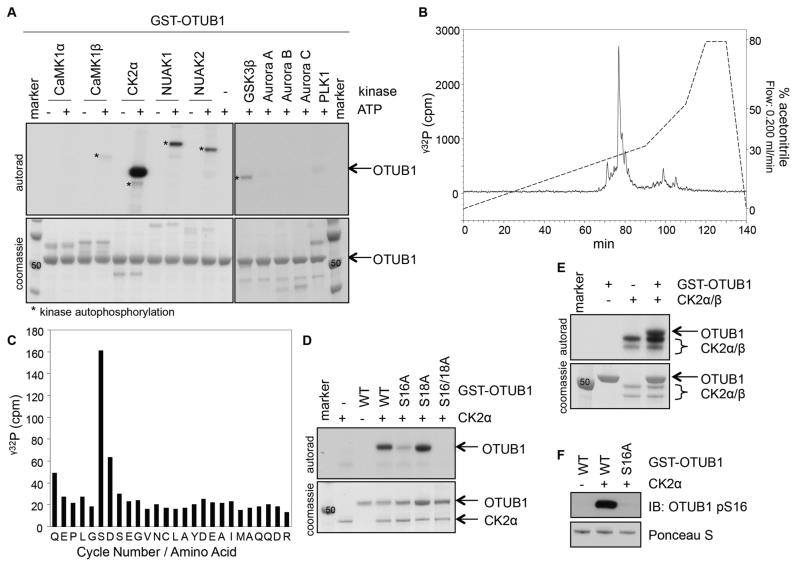
Figure 1. OTUB1 is phosphorylated by CK2 in vitro.
(A) Coomassie stain and autoradiography of SDS-PAGE after an in vitro kinase assay with various kinases and GST-OTUB1 as the substrate. (B) γ32P-release chromatograph of CK2-phosphorylated GST-OTUB1, which was digested with trypsin and resolved by HPLC on a C18 column on an increasing acetonitrile gradient as shown. (C) γ32P radioactivity release of the peak after each cycle of Edman degradation. (D) Coomassie stain and autoradiography of SDS-PAGE after an in vitro kinase assay with CK2α and wild-type (WT) or various mutants of GST-OTUB1. (E) As in (D), with the holoenzyme CK2α/β used as the kinase and GST-OTUB1 as the substrate. (F) Western blot (IB) of the phosphorylation of OTUB1 at Ser16 (pS16) after the in vitro kinase assay as in (D). Total GST-OTUB1 was detected with Ponceau S. All blots are representative of 3 independent experiments.