Fig. 6.
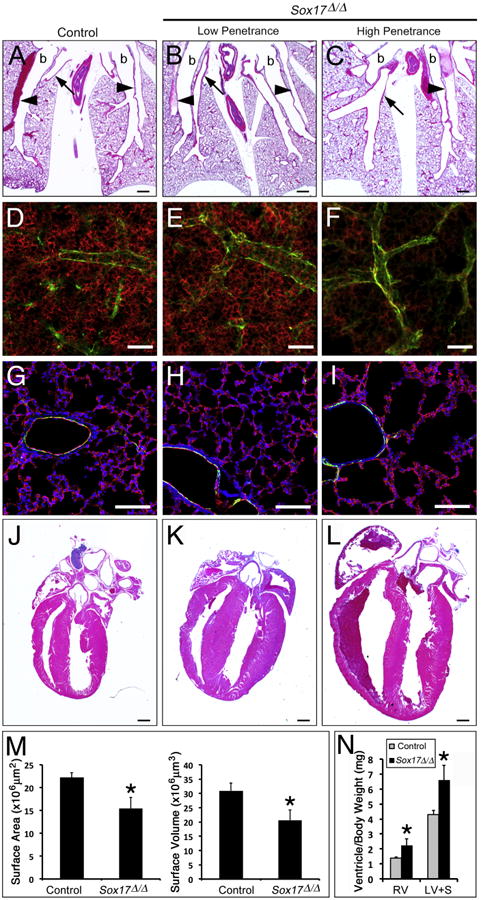
Postnatal Sox17Δ/Δ mice with pulmonary vascular abnormalites develop enlarged hearts. (A–C) Histological analysis of lungs from 2 week old control (A) and Sox17Δ/Δ (B–C) mice shows variable enlargement of pulmonary blood vessels. arrowheads, arteries; arrows, veins; b, bronchus. (D–I) Immunofluorescence staining for endomucin (red) and alpha-smooth muscle actin (green) was performed on lung sections from 2 week old control (D and G) and Sox17Δ/Δ (E and F, H and I) mice. (D-F) Whole mount staining of thick sections (150 μm) showed that the endomucin-positive microvascular network was variably reduced in the lungs of Sox17Δ/Δ mice. (G-I) Immunofluorescence staining of thin lung sections (10 μm) showed that paucity of the microvasculature was associated with alveolar simplification in severely affected Sox17Δ/Δ mice. (M) The area and volume of the pulmonary vascular network determined from endomucin staining were decreased in Sox17Δ/Δ mice. (J–L) Histological analysis of hearts from 2 week old control (J) and Sox17Δ/Δ (K and L) mice shows variable cardiac enlargement. (N) Ventricle weights were significantly increased in 2 week old Sox17Δ/Δ mice compared to controls. RV, right ventricle; LV+S, left ventricle and septum. The severity of pulmonary vascular abnormalities and heart enlargement in Sox17Δ/Δ mice were well correlated. Scale bars 500 μm (A–C and J–L); 100 μm (D–I). Asterisks indicated statistical significance as determined by Student's t-test (p ≤.05).
