Abstract
The hyperlipidemia accompanying infection has been attributed to production of tumor necrosis factor. This cytokine inhibits adipose tissue lipoprotein lipase, which could decrease clearance of lipoproteins. Infections also increase hepatic lipogenesis. We now have demonstrated that tumor necrosis factor-alpha stimulates lipid synthesis in vivo. 2 h after administration of tumor necrosis factor (25 micrograms/200 g), plasma triglycerides increase 2.2-fold and remain elevated for 17 h. Plasma cholesterol also increases, but this effect appears after 7 h. Tumor necrosis factor rapidly stimulates incorporation of tritiated water into fatty acids in the liver (1-2 h), which persists for 17 h. Also, tumor necrosis factor stimulates hepatic sterol synthesis. Of note, tumor necrosis factor treatment does not stimulate lipid synthesis in other tissues, including adipose tissue. Labeled fatty acids rapidly increase in the plasma, raising the possibility that stimulation of hepatic lipogenesis by tumor necrosis factor contributes to the hyperlipidemia of infection.
Full text
PDF


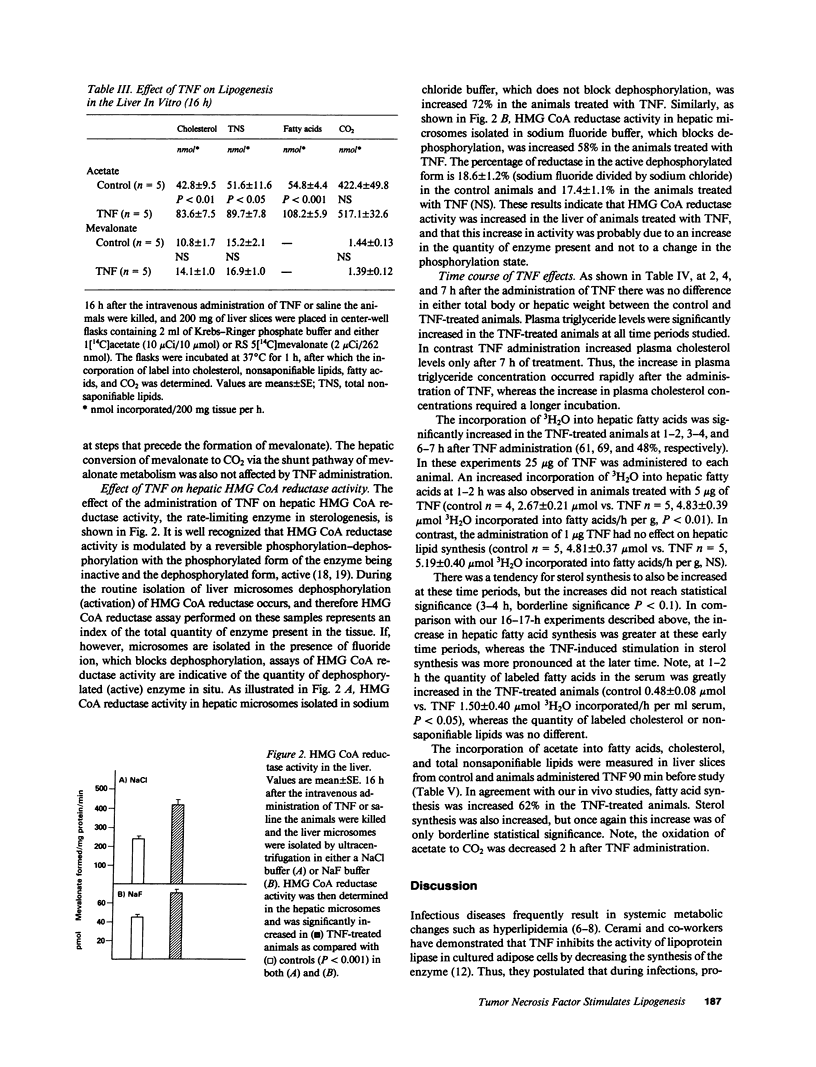


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beg Z. H., Brewer H. B., Jr Regulation of liver 3-hydroxy-3-methylglutaryl-CoA reductase. Curr Top Cell Regul. 1981;20:139–184. doi: 10.1016/b978-0-12-152820-1.50008-0. [DOI] [PubMed] [Google Scholar]
- Beisel W. R. Metabolic response to infection. Annu Rev Med. 1975;26:9–20. doi: 10.1146/annurev.me.26.020175.000301. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Greenwald D., Hulmes J. D., Chang M., Pan Y. C., Mathison J., Ulevitch R., Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985 Aug 8;316(6028):552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M., Spady D. K. Measurement of rates of cholesterol synthesis using tritiated water. J Lipid Res. 1984 Dec 15;25(13):1469–1476. [PubMed] [Google Scholar]
- Dinarello C. A., Mier J. W. Interleukins. Annu Rev Med. 1986;37:173–178. doi: 10.1146/annurev.me.37.020186.001133. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Moser A. H. Effect of lactation on cholesterol synthesis in rats. Am J Physiol. 1985 Aug;249(2 Pt 1):G203–G208. doi: 10.1152/ajpgi.1985.249.2.G203. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Wiley M. H., MacRae G., Lear S., Moser A. H., Zsigmond G., Siperstein M. D. De novo sterologenesis in the intact rat. Metabolism. 1983 Jan;32(1):75–81. doi: 10.1016/0026-0495(83)90160-9. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Wiley M. H., MacRae G., Moser A. H., Lear S. R., Siperstein M. D. The effect of diabetes mellitus on sterol synthesis in the intact rat. Diabetes. 1982 May;31(5 Pt 1):388–395. doi: 10.2337/diab.31.5.388. [DOI] [PubMed] [Google Scholar]
- Fiser R. H., Denniston J. C., Beisel W. R. Infection with Diplococcus pneumoniae and Salmonella typhimurium in monkeys: changes in plasma lipids and lipoproteins. J Infect Dis. 1972 Jan;125(1):54–60. doi: 10.1093/infdis/125.1.54. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Kaye D., O'Leary W. M. Serum lipids in infection. N Engl J Med. 1969 Nov 13;281(20):1081–1086. doi: 10.1056/NEJM196911132812001. [DOI] [PubMed] [Google Scholar]
- Gibson D. M., Ingebritsen T. S. Reversible modulation of liver hydroxymethylglutaryl CoA reductase. Life Sci. 1978 Dec 31;23(27-28):2649–2664. doi: 10.1016/0024-3205(78)90644-6. [DOI] [PubMed] [Google Scholar]
- Guckian J. C. Role of metabolism in pathogenesis of bacteremia due to Diplococcus pneumoniae in rabbits. J Infect Dis. 1973 Jan;127(1):1–8. doi: 10.1093/infdis/127.1.1. [DOI] [PubMed] [Google Scholar]
- Kaufmann R. L., Matson C. F., Beisel W. R. Hypertriglyceridemia produced by endotoxin: role of impaired triglyceride disposal mechanisms. J Infect Dis. 1976 May;133(5):548–555. doi: 10.1093/infdis/133.5.548. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Pekala P. H., Lane M. D., Cerami A. Lipoprotein lipase suppression in 3T3-L1 cells by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1982 Feb;79(3):912–916. doi: 10.1073/pnas.79.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Auger K. R., Robbins A. H., Birinyi L. K., Dinarello C. A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986 Aug;124(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Bank I., Handley D., Cassimeris J., Chess L., Stern D. Tumor necrosis factor/cachectin interacts with endothelial cell receptors to induce release of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1363–1375. doi: 10.1084/jem.163.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Patton J. S., Shepard H. M., Wilking H., Lewis G., Aggarwal B. B., Eessalu T. E., Gavin L. A., Grunfeld C. Interferons and tumor necrosis factors have similar catabolic effects on 3T3 L1 cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8313–8317. doi: 10.1073/pnas.83.21.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekala P. H., Kawakami M., Angus C. W., Lane M. D., Cerami A. Selective inhibition of synthesis of enzymes for de novo fatty acid biosynthesis by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2743–2747. doi: 10.1073/pnas.80.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekala P. H., Price S. R., Horn C. A., Hom B. E., Moss J., Cerami A. Model for cachexia in chronic disease: secretory products of endotoxin-stimulated macrophages induce a catabolic state in 3T3-L1 adipocytes. Trans Assoc Am Physicians. 1984;97:251–259. [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Torti F. M., Dieckmann B., Beutler B., Cerami A., Ringold G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]


