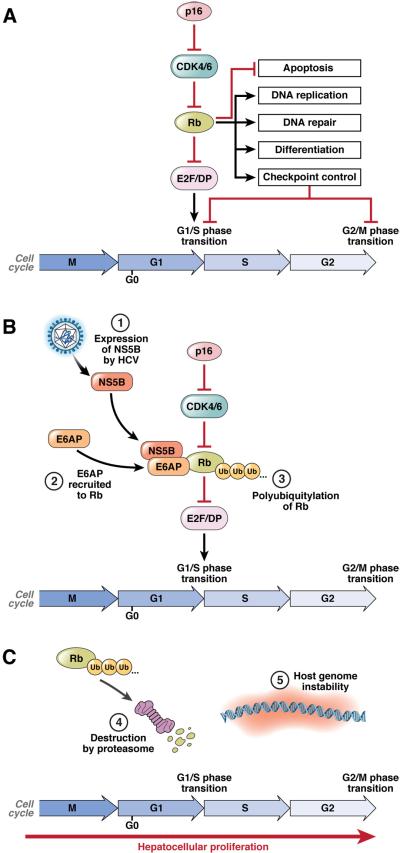
Figure 1. Disruption of the Rb pathway by HCV NS5B.
(A) Rb regulates multiple cellular processes, including cell cycle progression, apoptosis, DNA replication and repair, cellular differentiation and senescence. Rb binds to and represses E2F/DP transcription factor complexes. During the G1/S phase transition, Rb phosphorylation by cyclin-dependent kinases (CDKs) results in dissociation of Rb from E2F/DP complexes. The derepressed E2F/DP complexes are free to initiate transcription of S phase-specific genes, thus allowing cell cycle progression. (B) NS5B interacts with Rb in the cytoplasm of HCV-infected cells, and recruits the E3-ubiquitin ligase E6AP to facilitate ubiquitylation of Rb. (C) Ubiquitylation targets Rb for proteasome mediated degradation. Loss of Rb results in dysregulation of the many processes that Rb controls. For example, the unscheduled activation of E2F/DP-dependent gene expression can compromise cell cycle checkpoints that guard against chromosomal instability as well as induce p53-dependent apoptosis.