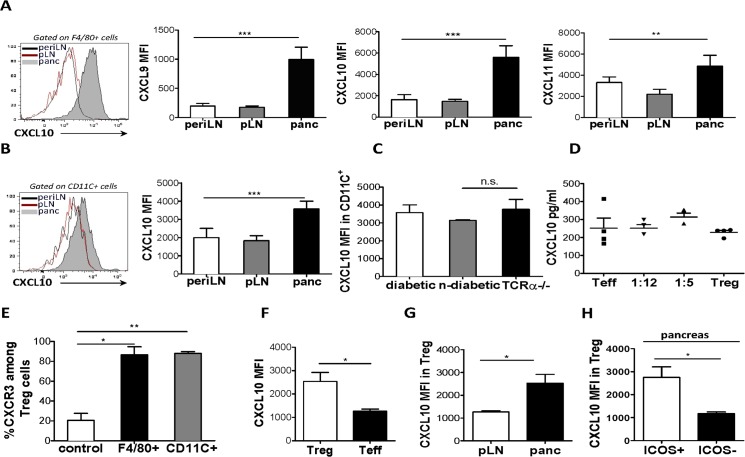
Fig 4. Resident leukocyte subsets in the pancreas express CXCR3-activating chemokines.
NOD.TCRα-/- mice initially received BDC2.5 CD4+ T cell (7.5X105) cells, and then cell suspensions from pancreas, draining pLN and peripheral LN of diabetic mice were examined for expression of CXCL9, CXCL10 and CXCL11 in F4/80+ (A) and CXCL10 in CD11c+ (B) cells was assessed by flow cytometry. (C) NOD.TCRα-/- mice were transferred with CD4+ T cells in order to induce T1D. Following adoptive transfer, glucose levels were measured daily in order to assess diabetes onset. Pancreatic cell suspensions from recipients were obtained and CXCL10 expression (MFI) was compared among CD11c+ cells from diabetic or non-diabetic recipients, as well as un-transferred (without T cell transfer) NOD.TCRα-/- mice. (D) NOD.TCRα-/- mice received Treg or Teff cells either alone or at the indicated Treg/Teff cell ratios. When mice receiving Teff cells alone became hyperglycemic (>33mmol/L), CXCL10 levels were assessed by ELISA in supernatants from pancreatic suspensions. (E), F4/80+ and CD11c+ (1X106/well) cells were seeded in triplicate in lower chambers, and CD4+ (1X106/well) cells in the upper chambers of 24 well Transwell plate. Following a 3 hour incubation period, the percent migrated CXCR3+ cells among ICOS+ Treg cells was compared between wells containing APCs and media alone (control). Cell suspensions of pancreas and draining LN of 4-week-old BDC2.5 mice were obtained and CXCL10 expression (MFI) was compared (F) between pancreatic Treg cells and Teff cells (G), between Trec cells at sites indicated, and between ICOS+ and ICOS+ Treg cells within pancreas (H). (4A-4C n = 6, 4D n = 4, 4E n = 2, 4F-4H n = 5).