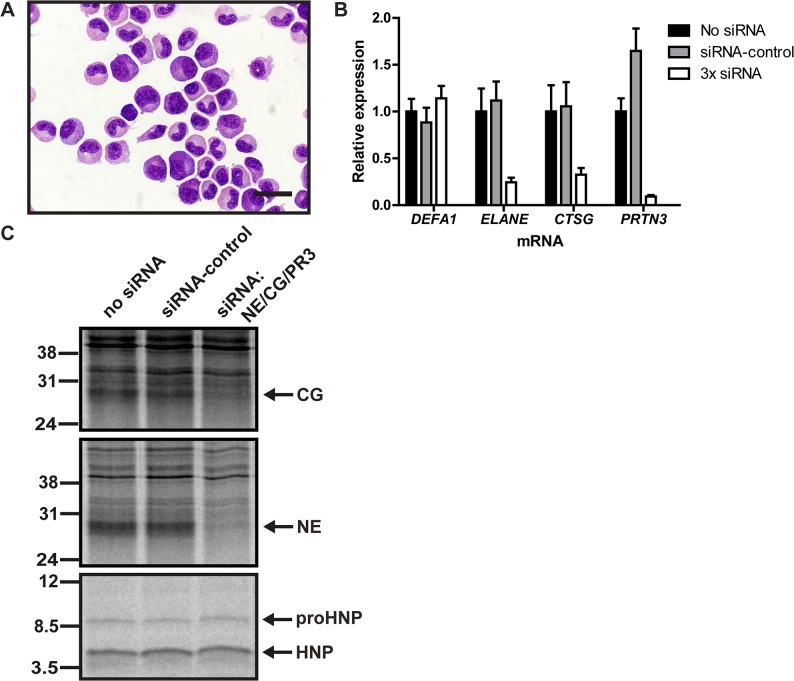
Fig 4. Biosynthesis of proHNP and HNP in human bone marrow.
(A) Human bone marrow cells were sedimented with dextran. Supernatant was laid on Lymphoprep and centrifuged at 400g for 30 minutes. Interphase cells were depleted of nongranulocytic cells by immunomagnetic sorting, spun onto slides and May-Grünwald Giemsa stained. Bar represents 20 μm. (B) Purified granulocytic precursors were electroporated with 3x siRNA (against neutrophil elastase (NE; ELANE), cathepsin G (CG; CTSG), and proteinase 3 (PR3; PRTN3)), control siRNA, or without siRNA and incubated for 24h in a humidified incubator with 5% CO2 at 37°C. Comparative quantification mRNA for DEFA1, ELANE, CTSG, and PRTN3 was performed by real-time PCR. Figure depicts expression levels relative to cells electroporated without siRNA. Bars represent means and lines represent standard deviation. (C) Transfected granulocyte precursors were pulsed with 35S-methionine/cysteine for 2 hours and chased overnight. Cell lysates and medium were immunoprecipitated with antibodies in the following order: anti-proHNP, anti-HNP, anti-CG, anti-NE, and anti-PR3. CG (top) and NE (mid) immunoprecipitates were analyzed by 12% SDS-PAGE and fluorography. Immunoprecipitation with anti-PR3 did not yield a specific PR3 band (data not shown). ProHNP and HNP immunoprecipitates were pooled and analyzed by 16% SDS-Tricine-PAGE and fluorography (bottom).