Abstract
Background
Epinephrine is a first-line drug for cardiopulmonary resuscitation, but its efficacy in the treatment of bupivacaine-induced cardiac toxicity is still in question. We hypothesized that epinephrine can reverse cardiac inhibition of bupivacaine by modulating ion flows through the ventricular myocyte membrane channels of rats. The aim of this study was to observe and report the effects of epinephrine on high-concentration bupivacaine-induced inhibition of sodium (INa), L-type calcium (ICa-L), and transient outward potassium (Ito) currents in the ventricular myocytes of rats.
Methods
The ventricular myocytes were isolated from Sprague-Dawley rats (250-300 g) by acute enzymatic dissociation. The whole-cell patch clamp technique was used to record the ion channel currents in single ventricular myocytes both before and after administration of medications.
Result
Administration of bupivacaine 100 μmol/L significantly reduced INa, (P < 0.05). However, administration of bupivacaine 100 μmol/L in conjunction with epinephrine 0.15 μg/ml had no effect in restoring INa to its previous state. Similarly, a sharp decline of ICa-L and Ito was observed after administration of bupivacaine 100 μmol/L (P < 0.05). In contrast to INa, ICa-L and Ito were significantly improved after the administration of the aforementioned combination of bupivacaine and epinephrine (P < 0.05).
Conclusion
Epinephrine can reverse high-concentration bupivacaine induced inhibition of ICa-L and Ito, but not INa. Thus, epinephrine’s effectiveness in reversal of bupivacaine-induced cardiac toxicity secondary to sodium channel inhibition may be limited.
Keywords: Bupivacaine, Epinephrine, Cardiac toxicity
Background
Epinephrine is a first-line drug for cardiopulmonary resuscitation. However, the extent of its effectiveness in bupivacaine-induced cardiac toxicity has remained unresolved [1-4]. Weinberg et al. [1] and Hiler et al. [2] reported that using epinephrine for resuscitation may increase myocardial oxygen consumption in the bupivacaine intoxication model of rats and rabbits. Its use in this setting may result in refractory ventricular fibrillation, pulmonary edema, acidosis, hypoxemia and other complications. On the other hand, Harvey et al. [3] claimed that epinephrine was necessary for circulatory recovery in their rabbit model of bupivacaine toxicity. Their claim was supported by work that demonstrated that epinephrine can accelerate hemodynamic recovery [4], reverse slowed action potential conduction, shorten action potential duration, and improve myocardial contractility in the face of increased plasma concentrations of bupivacaine [5].
Accidental intravascular injection of bupivacaine, over-absorption from peripheral tissues, and high plasma concentrations contribute to life-threatening cardiac rhythm disturbances. Previous studies suggested that the mechanism of bupivacaine-induced cardiac toxicity was secondary to its depression of the cardiac conduction system [6] through inhibition of the sodium channels [7,8] potassium channels [9,10] and calcium channels [11,12].
We hypothesized that epinephrine can reverse bupivacaine-induced cardiac inhibition by modulating INa, ICa-L, and Ito membrane channels of ventricular myocytes. We used the whole-cell patch clamp technique to observe the effect of epinephrine on these membrane channels in rat ventricular myocytes that were subjected to high-concentration bupivacaine-induced cardiac toxicity.
Methods
Isolated heart and preparation
Ths study was approved by the Animal Care and Use Committee of Wenzhou Medical College. Adult Male Sprague-Dawley rats, weighing 250 to 300 g, were anesthetized using 5% chloral hydrate (7 ml/kg intraperitioneal injection). The carotid artery is cut to allow rapid and thorough exsanguination. The hearts were rapidly removed intact with a short aortic remnant. While in this Tyrode solution the aorta is cannulated, one should make sure that there are no air bubbles trapped in the cannula. The cannulated heart is then mounted on a Langendorff perfusion apparatus with constant flow. After rapid excision, the heart was mounted in a modified Langendorff system and then perfused with the nominally Ca2 + -free Tytode solution (NaCl 137 mM、KCl 5.4 mM、MgCl2 1.0 mM、NaH2PO4 0.33 mM、HEPES 10 mM、Glucose 10 mM, and PH7.35 with NaOH) for 5 min at room temperature (20-25)°C. The perfusate reservoirs and column are glass jacketed, allowing the temperature to be maintained at 37°C by means of a recirculating water bath. All of the perfusion solutions are equilibrated with 100% oxygen. The heart is then retrogradely perfused with the nominally Ca2 + -free Tyrode solution which causes cessation of the heartbeat until the blood is washed out. This was followed by perfusion with enzyme solution, containing 1 mg/ml collagenase (Sigma, typeI) in nominally Ca2 + -free Tytode solution, followed for 10 ~ 15 min. The softened heart was removed from the column, and the left ventricle was dissected in modified KB (KCl 40 mM, KH2PO4 20 mM, MgSO4 3.0 mM, KOH 80 mM, Glutamate 50 mM, Taurine 20 mM, HEPES 10 mM, glucose 10 mM, EGTA 0.5 mM, and pH 7.35 with KOH). The cells were maintained in modified KB solution and stabilized at room temperature for 1 hour.
Equipments and methods
An EPC-10 patch clamp amplifier (HEKA, Germany) was used for application of the whole-cell patch clamp in the ventricular myocytes. Pulse stimulation and data acquisition were recorded by Pulse 8.0 software (HEKA, Germany). Patch pipettes were pulled from glass tubing with a 1.5 mm outer diameter (SUTTER, USA) by the use of micro-electrodes (P-97, SUTTER, USA), and the tip was heated to give a resistance of 1.5-2.5 MΩ when filled with the specific, appropriate solution (see below). Using an inverted microscope, microelectrodes were directed to the ventricular myocytes by a three-dimensional micromanipulator (MP-285, SUTTER, USA). A Giga-seal was formed after vacuum suction. The patch membrane was broken after fast capacitance compensation by the provision of additional suction with subsequent construction of a whole-cell recording. To reduce the instantaneous current charging/discharging, and to minimize clamping errors, the slow capacitance compensation and series resistance compensation were settled at 70% - 80%. Leakage currents were subtracted by the P/4 method.
Electrophysiology
1. For recording INa, the external solution was composed of the following, in mM: Choline-Cl 120, NaCl 20, MgCl2 1.0, HEPES 5, Glucose 10, CsCl 4.6, pH 7.35 with CsOH. the internal solution was composed of the following, in mM: CsCl 120、NaCl 10、MgCl2 1.0、Na2ATP 5.0、EGTA 10、HEPES 10, pH 7.3 with CsOH.
The potential was held at -90 mV, INa was evoked by 25 ms, and accompanied by -30 mV square-wave depolarizing pluses. The stimulation program of current density-voltage curve was as follows: under a holding potential (Vh) of -90 mV, the step clamp voltage (Vs) was stimulated from -90 mV to +50 mV by a step of 10 mV with a 50 ms duration, and a 0.2 Hz stimulation frequency.
2. For recording ICa-L, the external solution was composed of the following, in mM: Choline-Cl 140, MgCl2 1.0, CaCl2 2.0, HEPES 5, Glucose 10, CsCl 4.6, TEA-Cl 10, pH 7.35 with CsOH. the internal solution was composed of the following, in mM: CsCl 120、MgCl2 1.0、MgATP 5.0、EGTA 10、HEPES 10 TEA-Cl 10, pH 7.3 with CsOH.
The potential was held at -40 mV, and ICa-L was evoked by 150 ms, accompanied by 0 mV square-wave depolarizing pluses. The stimulation program of current density-voltage curve was as follows: under a Vh of -40 mV, the Vs was stimulated from -40 mV to +50 mV by a step of 10 mV with a 250 ms duration, and a 0.2 Hz stimulation frequency.
3. For recording Ito, the external solution was composed of the following, in mM: NaCl 137, KCl 5.4, CaCl2 1.8, MgCl2 1.0, NaH2PO4 0.33, HEPES 10, Glucose 10, CdCl2 0.3, pH t7.35 with NaOH.the internal solution was composed of the following, in mM: KCl 140、MgCl2 1.0、K2ATP 5.0、EGTA 10、HEPES 5,pH 7.3 with KOH.
The potential was held at -90 mV, Ito was evoked by 20 ms, 50 mV square-wave depolarizing pluses. The stimulation program of current density-voltage curve was: under a Vh of -90 mV, and INa was eliminated by 20 ms, with -40 mV depolarizing pluses; the Vs was stimulated from -40 mV to +50 mV by a step of 10 mV with a 400 ms duration and a 0.2 Hz stimulation frequency.
A control curve of I-V was collected before drug perfusion (T0). Then 100 umol/L bupivacaine and the mixture of 100 umol/L bupivacaine and 0.15 μg/ml epinephrine were added into the reservoirs respectively from superfusion systerm (DADVC-8PP,ALA SCIENTIFIC, USA). The DAD-VC systems go out with a Micromanifold consisting of 8 tubes of polyamide coated quartz glass of 100 um ID. The Micromanifold enables up to 8 solutions from the reservoirs to flow into a small common space of less than 1ul. The Micromanifold with a micromanipulator can easily be moved around the cell preparation and pointed at the target cell. The user must be careful to aim the output so that it completely bathes the cell under study. So during the study there is no motion of the output tip to be dealt with and there is no need to have all the solutions flowing out and contaminating the preparation solution during an experiment. After the cell surface perfusion with 100 μmol/L bupivacaine for 10 seconds. The peak current and I-V curve were recorded (T1). The microperfusion tube was then swithed by another reservoir prefilled with 100 μmol/L bupivacaine and 0.15 ug/ml epinephrine after the model was successfully made by 100 μmol/L bupivacaine. The peak current and I-V curve were then recorded at the time (T2) when the cells’ surface were perfusion with 100 μmol/L bupivacaine and 0.15 ug/ml epinephrine for 10 seconds.
To eliminate the error among cells, the size of the ion currents was represented by the current density, which was the ratio of current intensity and cell membrane capacitance (pA/pF). Data were stored in the hard disk for the measurement and analysis. Raw current data were analyzed and measured by pulse 8.0.
Statistical analysis
All data were analyzed using SPSS 13.0 and presented as mean ± SD. The results were analyzed with a paired t test, the P value were using the Bonferroni correction,. A P value < 0.05 was considered statistical significance. The graphs were performed by Origin 8.0.
Results
The various ion currents could be obtained from the fresh isolated ventricular myocytes, which indicated that the cells had good electrophysiological properties.
Effects of epinephrine on bupivacaine-induced inhibition of sodium currents in ventricular myocytes of rats
Sodium currents: The INa at T0, T1, and T2 was (-8.3 ± 0.9) (pA/pF), (-2.2 ± 0. 6) (pA/pF), and (-2.3 ± 0.7) (pA/pF), respectively (n = 5, T1vs T0, P < 0.001; T2vs T0, P < 0.001; T2vs T1, P =0.322) (Table 1). Figure 1 depicts the effects of bupivacaine alone and in combination with epinephrine on the INa trace.
Table 1.
Bupivacaine alone and combination with epinephrine affect the channels current-density
| Channel current | Cell number | TO (pA/pF) | T1 (pA/pF) | T2 (pA/pF) |
|---|---|---|---|---|
| INa | 5 | −8.3 ± 0.9 | −2.2 ± 0. 6 | −2.3 ± 0.7 |
| ICa-L | 6 | −7. 8 ± 0.7 | −2.0 ± 0.6 | −4.9 ± 0.9 |
| Ito | 6 | 23 ± 5 | 15 ± 3 | 26 ± 8 |
Data were shown as mean ± SD. T0 = before the bupivacaine perfusion; T1 = after the perfusion of the 100 μmol/L bupivacaine; T2 = after the perfusion of the 100 μmol/L bupivacaine with 0.15 μg/ml epinephrine.
INa: T1 vs T0, P < 0.001; T2 vs T1, P >0.05.
ICa-L: T1 vs T0, P < 0.001; T2 vs T1, P < 0.001.
Ito: T1vs T0, P < 0.05; T2vs T1, P < 0.05.
Figure 1.
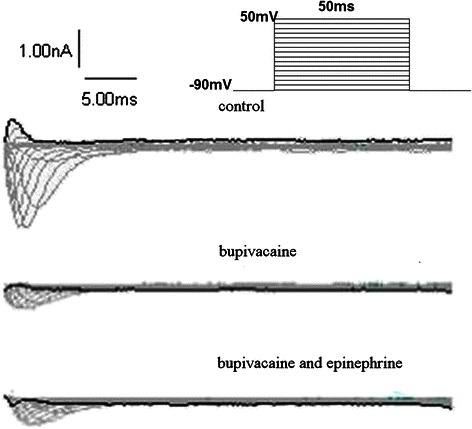
Representative INa traces obtained from ventricular myocytes groups: control, bupivacaine 100 μmol/L alone, and bupivacaine with epinephrine 0.15 μg/ml. Family of current traces obtained by applying a series of 50 ms pulses ranging from -90 mV to 50 mV with a holding potential of -90 mV.
Sodium channel current density-voltage curve: The activation potential of INa was -60 mV, with a peak potential of -30 mV and a reverse potential of +20 mV. Bupivacaine 100 μmol/L present in the perfusate lead to the INa decrease, and an upper shift of the current density-voltage curve, without changing the peak potential, activation potential, or the reversal potential. Administration of bupivacaine 100 μmol/L and epinephrine 0.15 μg/ml did not effect any change in the INa current density-voltage curve (Figure 2).
Figure 2.
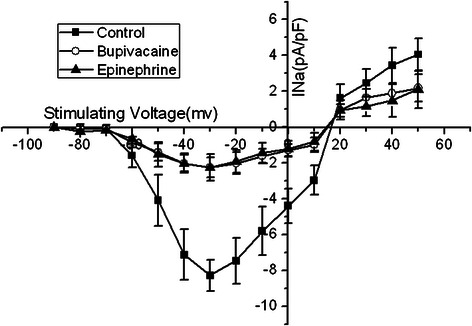
The INa current-voltage relationship in the absence of bupivacaine, with bupivacaine 100 μmol/L alone, and bupivacaine 100 μmol/L in combination with epinephrine 0.15 μg/ml. Bupivacaine 100 μmol/L present in the perfusate contributed to an upper shift of the current density-voltage curve. Administration of 100 μmol/L bupivacaine and epinephrine 0.15 μg/ml did not effect any change in the curve.
Effects of epinephrine on bupivacaine-induced inhibition of L-type calcium currents in ventricular myocytes of rats
L-type calcium currents: The ICa-L at T0, T1, and T2 was (-7.8 ± 0.7) (pA/pF), (-2.0 ± 0. 6) (pA/pF), and (-4.9 ± 0.9) (pA/pF), respectively (n = 6, T1vs T0, P < 0.001; T2vs T0, P < 0.001; T2vs T1, P < 0.001). Figure 3 depicts the effects of bupivacaine 100 μmol/L alone and in combination with epinephrine 0.15 μg/ml on the ICa-L trace.
Figure 3.
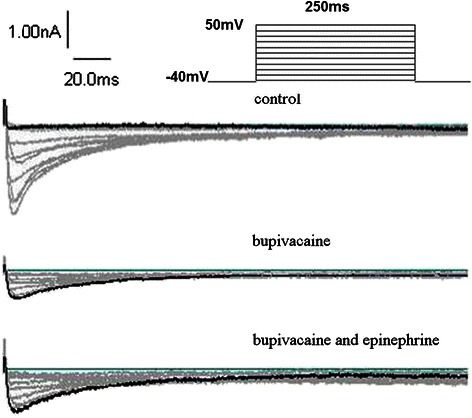
Representative ICa traces obtained from ventricular myocytes groups: control, bupivacaine 100 μmol/L alone, and bupivacaine 100 μmol/L in combination with epinephrine 0.15 μg/ml. Family of current traces obtained by applying a series of 250 ms pulses ranging from -40 mV to 50 mV with a holding potential of -40 mV.
ICa-L was -30 mV, with a peak potential of 0 mV and a reverse potential of +40 mV. Bupivacaine 100 μmol/L present in the perfusate contributed to the upper shift of the current density - voltage curve without changing the activation potential and the curve shape. However, bupivacaine 100 μmol/L in combination with epinephrine 0.15 μg/ml increased the ICa-L current, and moved the current density-voltage curve of ICa-L downward (Figure 4).
Figure 4.
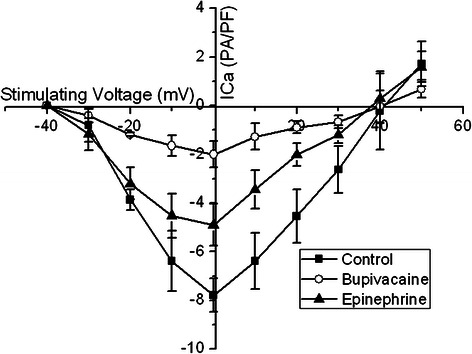
The ICa-L current-voltage relationship in the absence of bupivacaine, with bupivacaine 100 μmol/L alone, and bupivacaine 100 μmol/L in combination with epinephrine 0.15 μg/ml. Bupivacaine 100 μmol/L present in the perfusate contributed to an upper shift of the current density - voltage curve without changing the activation potential and the curve shape. However, bupivacaine 100 μmol/L in combination with epinephrine 0.15 μg/ml increased the ICa-L current and moved the curve of ICa-L downward.
Effects of epinephrine on bupivacaine-induced inhibition of transient outward potassium currents in ventricular myocytes of rats
Transient outward potassium currents: The current density of Ito at T0, T1, and T2 was (23 ± 5) (pA/pF), (15 ± 3) (pA/pF), and (26 ± 8) (pA/pF), respectively (n = 6, T1vs T0, P =0.013; T2vs T0, P = 0.161; T2vs T1, P =0.003). Figure 5 depicts the effects of bupivacaine 100 μmol/L alone, and in combination with epinephrine 0.15 μg/ml on the Ito trace.
Figure 5.
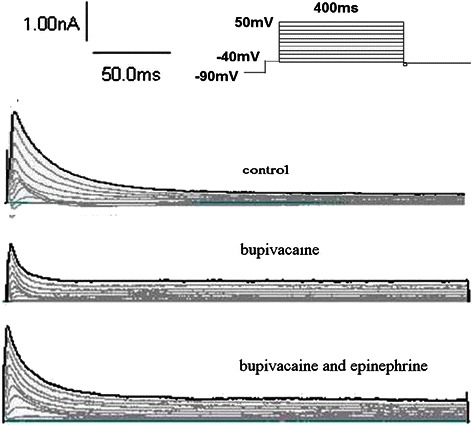
Representative Ito traces obtained from ventricular myocyte groups: control, bupivacaine 100 μmol/L alone, and bupivacaine 100 μmol/L in combination with epinephrine 0.15 μg/ml. Family of current traces obtained by applying a series of 20ms test pulses of -40 mV to eliminate current through Na channels, which was then evoked by a series of 400 ms pulses ranging from -40 mV to 50 mV with a holding potential of -90 mV.
Transient outward potassium channel currents density-voltage curves: The activation potential of Ito was at -40 mV with the higher voltage the current was increased, and the outward rectification characteristics were evident. Bupivacaine 100 μmol/L without epinephrine decreased Ito. On the other hand, Ito increased in response to the bupivacaine 100 μmol/L in combination with epinephrine 0.15 μg/ml. However, both their rectifier characteristics were not changed (Figure 6).
Figure 6.
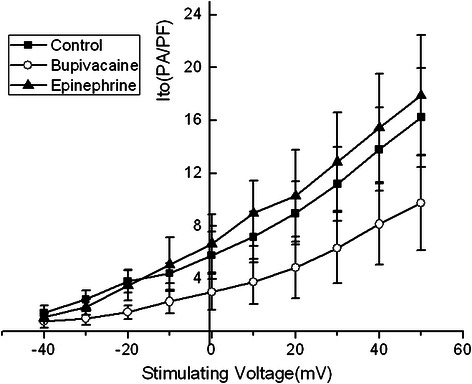
The Ito current-voltage relationship in the absence of bupivacaine, with 100 μmol/L bupivacaine alone, and 100 μmol/L bupivacaine in combination with epinephrine 0.15 μg/ml. Bupivacaine 100 μmol/L without epinephrine decreased Ito. On the other hand, Ito increased in response to the bupivacaine 100 μmol/L in combination with epinephrine 0.15 μg/ml.
Discussion
This study demonstrates that epinephrine can reverse bupivacaine-induced inhibition of calcium channels and the transient outward current of the potassium channels in the ventricular myocytes of rats, but with no significant effect on improving ionic flow in the sodium channels, i.e., reversing its inhibition.
Epinephrine is a potent heart stimulant. Currently, the American Heart Association recommends use of epinephrine during CPR [13], as a first-line drug for treating cardiac arrest, however, the use of epinephrine for bupivacaine-induced cardiac toxicity remains controversial [1-4].
The essence of bupivacaine-induced cardiac toxicity involves its ability to block myocardial cell ion channels. Rapid intravenous injection of a clinical dose of bupivacaine may lead to a cardiac arrest secondary to high plasma concentrations of bupivacaine that reach ≥ 100 μmol/L in a short period of time [14]. Thereby it reducing the amplitude of the myocardial action potential, shortening the action potential duration [12], and significantly inhibiting the myocardial sodium [6], potassium [8] and calcium [11] channel function. Moreover, while our results confirm these findings, we also have electrophysiological confirmation that epinephrine alone may be of little use in regard to sodium channel “resuscitation” as opposed to its ability to aid the calcium or potassium channels. Discussion of our study’s impact on the sodium, calcium, and potassium channels is warranted at this juncture.
INa facilitates the depolarization of the action potential (phrase 0), which affects excitability, refractoriness and conductivity of myocardial cells. Depolarizing of this action potential is impeded by bupivacaine, and its cardiac toxicity is directly related to the impaired flow Na+ inflow, which results in conduction disturbances and a series of cardiac arrhythmias [6] that may lead to cardiac arrest. A plasma bupivacaine concentration of 100 μmol/L blocks 73% of the sodium current. Catecholamine impact on the Na+ currents remains controversial. Wang [15] and Gintant [16] indicate that β-receptor agonists increase the sodium current, while others report that high concentrations of isoproterenol (a ß agonist) can produce inhibition of Na+ currents [17]. Our results indicate that epinephrine does not effectively support the recovery of ionic flow in the bupivacaine-inhibited sodium channels. This suggests that the use of epinephrine in reversing bupivacaine-induced cardiac toxicity may have limitations.
ICa-L plays an important role in the formation of the action potential plateau by increasing intracellular calcium and myocardial contraction. There is a concentration-dependent relationship between bupivacaine and its inhibition of L-type calcium channels in rat ventricular myocytes. A bupivacaine concentration of 100 μmol/L is roughly equivalent to the IC50 inhibition of ICa-L [11]. The inhibitory effect of ICa-L in our study was 50% higher than that reported in the literature and this may be related to our method of drug perfusion. We ensured that the bupivacaine concentration on the surface of the cell membrane reached 100 μmol/L instantly by using a direct microperfusion system. The cells in this case lacked a process of gradual adaptation, which may have enhanced the inhibitory effect on the channels. This situation may be analogous with the clinical picture of acute bupivacaine toxicity. Although β-adrenergic agonists can augment the frequency of ionic channel opening and current passage through the L-type calcium channel [18,19], there are no published reports as to whether epinephrine can reverse bupivacaine-induced inhibition of calcium channels. In our study bupivacaine suppressed the calcium current, but this current was partially restored by epinephrine. Thereby contributing to improved myocardial contractility and physiologic recovery.
With the rapid activation and inactivation in the early repolarization period of the action potential, Ito exhibited a concentration-dependent inhibition by bupivacaine on ventricular myocytes [8]. In humans, the inhibition of Ito prolongs the repolarization of the myocardial action potential and reduces the excitation-contraction coupling efficiency of the myocardial cells. Thus weakening the cardiac contractility by impairing regulation of the sarcoplasmic reticulum’s calcium load, releasing and influencing the function of the L-type calcium channel, and impairing the sodium calcium exchange mechanism. Additionally, the Ito of rat ventricular myocytess can be partially inhibited by α1-adrenergic receptor agonists [20]. However, our study demonstrates that epinephrine can reverse the inhibition of Ito induced by bupivacaine. Through the enhancement of the calcium current and an increased intracellular Ca2+ concentration secondary to activated β adrenergic receptors, there is a resultant increase in cardiac contractility through the indirect recovery of calcium-dependent Ito [21].
In this study we used the dose of epinephrine 0.15 μg/ml, which was based on our previous work demonstrating that isolated, arrested rats hearts recovered with this particular dose [22]. Additionally, we added bupivacaine 100 μmol/L to avoid bupivacaine elution in the process of extracellular fluid perfusion on the cell’s surface in order to simulate a setting where there is a critical plasma concentration of bupivacaine that induces cardiac toxicity.
The results/conclusions of our study are limited by the fact that we did not observe any reversal effect of epinephrine on the inhibition of sodium channel induced by different concentrations of bupivacaine, and we did not observe any reversal of the inhibitory effect of differing concentrations of epinephrine on the ion channels.
Conclusions
Epinephrine can reverse high-concentration bupivacaine-induced inhibition of calcium channels and the transient outward potassium current channels in the ventricular myocytes of rats, whereas it has no significant effect on improving sodium channel ionic flows. Thereby providing no augmentation of its action potential depolarization. These findings support the position that the use of epinephrine alone in the resuscitation of bupivacaine-induced cardiac toxicity/arrest may be of limited value in that it may produce a less than optimal result. It may be that adjunctive therapies/medications are needed to augment the use of epinephrine in the setting of bupivacaine-induced cardiac toxicity/arrest.
Acknowledgements
This study has been supported by natural science foundation of Zhejiang province, China (No. LY13H090008), No other financial support was received. We acknowledge Thomas J. Papadimos, who advised on English phrasing in some versions of the manuscript and provided the final edit.
Abbreviations
- KB
Kraft-Bruhe
- 4-AP
4-Aminopyridine
- TEA-Cl
Tetraethylammonium chloride
- BSA
Bovine Serum Albumin
- HEPES
N-2-hydroxyethylpiperazine-N-ethane-sulphonicacid
- ICa-L
L-type calcium current
- ICa-T
T-type calcium current
- INa
Sodium current
- IK1
Inward rectifier potassium current
- Ito
Transient outward potassium current
- Vh
Membrane holding potential
- Vrev
Reverse potential
- Vs
Step holding potential
- IC50
Half-maximal inhibitory concentration
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
FL responsible for the study design and the execution of the study, including data analysis and manuscript preparation. BW and YD participated in the study and in manuscript preparation. YW and HC participated in the study and in data analysis. FX and ZJ participated in the execution of the study. XX responsible for the study design and coordinated the team efforts. All authors have read and approved the final manuscript.
Contributor Information
Fuli Liu, Email: 903321930@qq.com.
Bingjing Wu, Email: doudou198251@sina.com.
Yongjun Du, Email: 75140942@qq.com.
Yiquan Wu, Email: wuyiquanspring@126.com.
Hongfei Chen, Email: chhfei@126.com.
Fangfang Xia, Email: helenxia4271@yahoo.com.cn.
Zhousheng Jin, Email: jzs185190370@163.com.
Xuzhong Xu, Email: xuzhong@263.net.
References
- 1.Weinberg GL, Greqorio GD, Ripper R, Ripper R, Kelly K, Massad M, et al. Resuscitation with lipid versus epinephrine in a rat model of bupivacaine overdose. Anesthesiology. 2008;108(5):907–13. doi: 10.1097/ALN.0b013e31816d91d2. [DOI] [PubMed] [Google Scholar]
- 2.Hiller DB, Gregorio GD, Ripper R, Kelly K, Massad M, Edelman L, et al. Epinephrine impairs lipid resuscitation from bupivacaine overdose: a threshold effect. Anesthesiology. 2009;111(3):498–505. doi: 10.1097/ALN.0b013e3181afde0a. [DOI] [PubMed] [Google Scholar]
- 3.Harvey M, Cave G, Prince G, Lahner D. Epinephrine injection in lipid-based resuscitation from bupivacaine-induced cardiac arrest: transient circulatory return in rabbits. Anesth Analg. 2010;111(3):791–6. doi: 10.1213/ANE.0b013e3181e66050. [DOI] [PubMed] [Google Scholar]
- 4.Heavner JE, Pitkanen MT, Shi B, Rosenberg PH. Resuscitation from bupivacaine-induced asystole in rats: comparison of different cardioactive drugs. Anesth Analg. 1995;80(6):1134–9. doi: 10.1097/00000539-199506000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Azuma M, Yamane M, Tachibana K, Morimoto Y, Kemmotsu O. Effects of epinephrine and phosphodiesterase III inhibitors on bupivacaine-induced myocardial depression in guinea-pig papillary muscle. Br J Anaesth. 2003;90(1):66–71. doi: 10.1093/bja/aeg025. [DOI] [PubMed] [Google Scholar]
- 6.Shibuya N, Momose Y, Ito Y. Effects of bupivacaine on contraction and membrane potential in isolated canine papillary muscles. Pharmacology. 1993;47(3):158–66. doi: 10.1159/000139093. [DOI] [PubMed] [Google Scholar]
- 7.Clarkson CW, Hondeghem LM. Mechanism for bupivacaine depression of cardiac conduction: fast block of sodium channels during the action potential with slow recovery from block during diastole. Anesthesiology. 1985;62(4):396–405. doi: 10.1097/00000542-198504000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Valenzuela C, Snyders DJ, Bennett PB, Tamargo J, Hondeghem LM. Stereoselective block of cardiac sodium channels by bupivacaine in guinea pig ventricular myocytes. Circulation. 1995;92(10):3014–24. doi: 10.1161/01.CIR.92.10.3014. [DOI] [PubMed] [Google Scholar]
- 9.Castle NA. Bupivacaine inhibits the transient outward K+ current but not the inward rectifier in rat ventricular myocytes. J Pharmacol Exp Ther. 1990;255(3):1038–46. [PubMed] [Google Scholar]
- 10.Courtney KR, Kendig JJ. Bupivacaine is an effective potassium channel blocker in heart. Biochim Biophys Acta. 1988;939(1):163–6. doi: 10.1016/0005-2736(88)90058-2. [DOI] [PubMed] [Google Scholar]
- 11.Zapata-Sudo G, Trachez MM, Sudo RT, Nelson TE. Is comparative cardiotoxicity of S(-) and R(+) bupivacaine related to enantiomer-selective inhibition of L-type Ca(2+) channels. Anesth Analg. 2001;92(2):496–501. doi: 10.1213/00000539-200102000-00040. [DOI] [PubMed] [Google Scholar]
- 12.Rossner KL, Freese KJ. Bupivacaine inhibition of L-type calcium current in ventricular cardiomyocytes of hamster. Anesthesiology. 1997;87(4):926–34. doi: 10.1097/00000542-199710000-00028. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg G. Lipid infusion resuscitation for local anesthetic toxicity: proof of clinical efficacy. Anesthesiology. 2006;105(1):7–8. doi: 10.1097/00000542-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Ohmura S, Kawada M, Ohta T, Yamamoto K, Kobayashi T. Systemic toxicity and resuscitation in bupivacaine-, levobupivacaine-, or ropivacaine-infused rats. Anesth Analg. 2001;93(3):743–8. doi: 10.1097/00000539-200109000-00039. [DOI] [PubMed] [Google Scholar]
- 15.Wang HW, Yang ZF, Zhang Y, Yang JM, Liu YM, Li CZ. Beta-receptor activation increases sodium current in guinea pig heart. Acta Pharmacol Sin. 2009;30(8):1115–22. doi: 10.1038/aps.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gintant GA, Liu DW. Beta-adrenergic modulation of fast inward sodium current in canine myocardium. Syncytial preparations versus isolated myocytes. Circ Res. 1992;70(4):844–50. doi: 10.1161/01.RES.70.4.844. [DOI] [PubMed] [Google Scholar]
- 17.Kirstein M, Eickhorn R, Kochsiek K, Langenfeld H. Dose-dependent alteration of rat cardiac sodium current by isoproterenol: results from direct measurements on multicellular preparations. Pflugers Arch. 1996;431(3):395–401. doi: 10.1007/BF02207277. [DOI] [PubMed] [Google Scholar]
- 18.Christ T, Galindo-Tovar A, Thoms M, Ravens U, Kaumann AJ. Inotropy and L-type Ca2+ current, activated by beta1- and beta2-adrenoceptors, are differently controlled by phosphodiesterases 3 and 4 in rat heart. Br J Pharmacol. 2009;156(1):62–83. doi: 10.1111/j.1476-5381.2008.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callewaert G, Cleemann L, Morad M. Epinephrine enhances Ca2+ current-regulated Ca2+ release and Ca2+ reuptake in rat ventricular myocytes. Proc Natl Acad Sci U S A. 1988;85(6):2009–13. doi: 10.1073/pnas.85.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tohse N, Nakaya H, Hattori Y, Endou M, Kanno M. Inhibitory effect mediated by alpha 1-adrenoceptors on transient outward current in isolated rat ventricular cells. Pflugers Arch. 1990;415(5):575–81. doi: 10.1007/BF02583508. [DOI] [PubMed] [Google Scholar]
- 21.Koster OF, Szigeti GP, Beuckelmann DJ. Characterization of a [Ca2+]i-dependent current in human atrial and ventricular cardiomyocytes in the absence of Na + and K+ Cardiovasc Res. 1999;41(1):175–87. doi: 10.1016/S0008-6363(98)00202-8. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Xia Y, Chen Y, Wang Q, Shi T, Wang F, et al. The comparative effects of lipid, epinephrine,and their combination in the reversal of bupivacaine-induced asystole in the isolated rat heart. Anesth Analg. 2012;114(4):886–93. doi: 10.1213/ANE.0b013e3182166a0a. [DOI] [PubMed] [Google Scholar]


