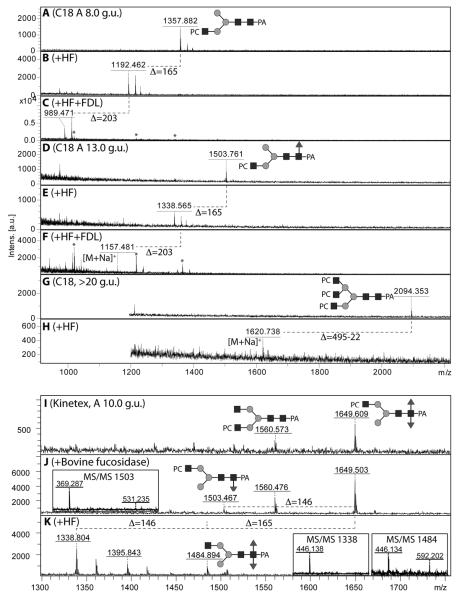
Figure 9. Chemical and enzymatic treatments of pyridylamino-labelled phosphorycholine-modified N-glycans.
Selected C18 fractions (A, D, G) were subject to hydrofluoric acid treatment in order to demonstrate the presence of phosphodiesters (loss of one phosphorylcholine is indicated by a loss of 165 Da; B, E, H); in order to determine on which arm the HexNAc1PC1 motif was present, two of these fractions were subsequently subject to treatment with the specific FDL hexosaminidase (C,F) to determine whether a GlcNAc was released from the α1,3-arm. Furthermore, a specific Kinetex-fractionated glycan containing a putative phosphorylcholine-modified difucosylated N-glycan (I, m/z 1649, for MS/MS see Figure 8G) was subject to fucosidase and hydrofluoric acid treatments (J,K); the latter resulted in full removal of the phosphorylcholine moiety and incomplete removal of an α1,3-fucose (see insets for relevant MS/MS of the digestion products); the co-eluting m/z 1560 glycan (Hex3HexNAc4PC1) is also sensitive to this treatment (product of m/z 1395 in panel K).