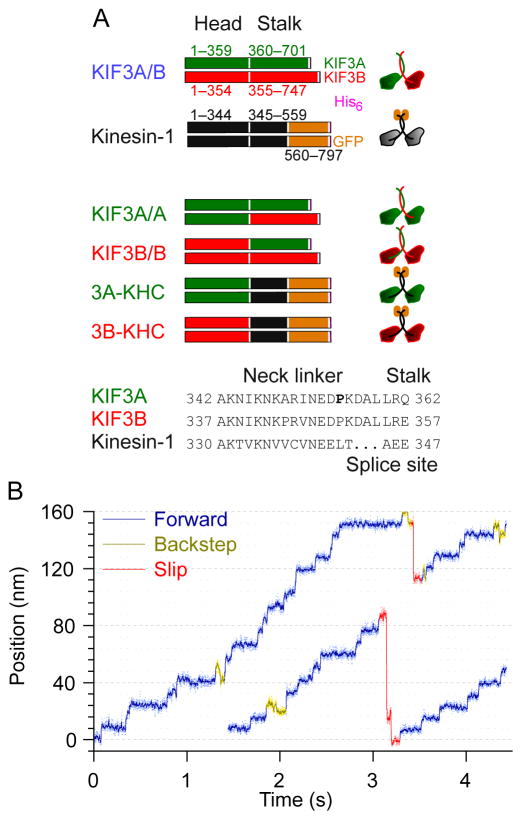
Figure 1. KIF3A/B stepping against hindering load in an optical force clamp.
(A) Recombinant kinesin constructs used in the study. KIF3A/B (blue label) consists of the full-length KIF3A (green) and KIF3B (red) sequences fused to a C-terminal His6 tag (pink). Kinesin-1 (black label) is a truncated DmKHC construct (black) fused to the GFP sequence (orange) and a His6 tag. Homodimeric mutants were generated by joining the KIF3A or KIF3B motor domains to the stalks of KIF3A/B or Kinesin-1. The splice site was the junction between the neck linker and stalk for the respective motors. Two additional mutants of 3A-KHC were also created: 3A-KHCP>A replaces the KIF3A NL proline (P, bold) by alanine, and 3A-KHCP>A,ΔDAL, which carries the identical mutation, together with a deletion of the three C-terminal neck-linker residues (DAL, underscored).
(B) Representative records of single-molecule movement for KIF 3A/B (4 pN hindering load, 5 μM ATP) displaying forward steps of 8 nm (blue), backsteps of 8 nm (olive) and slips of variable distance (red).