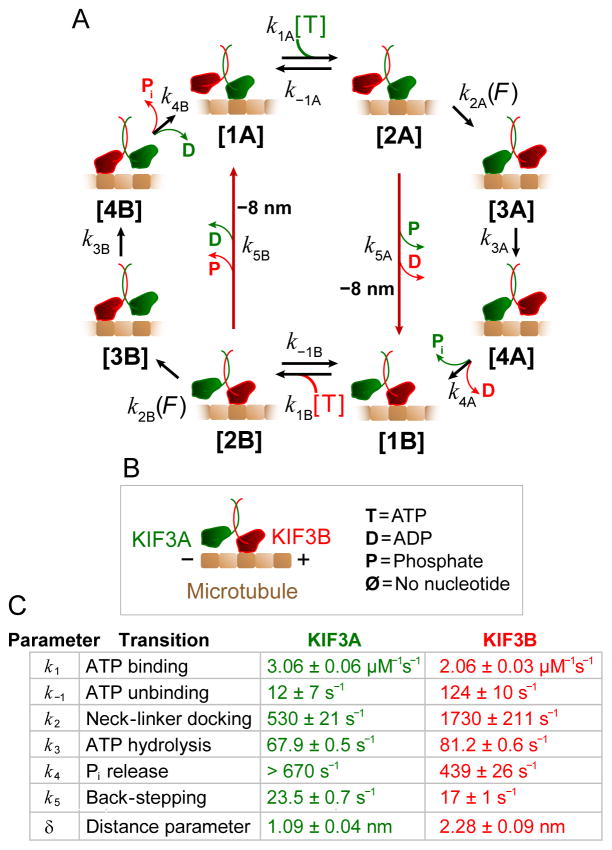
Figure 3. Modeling the KIF3A/B mechanochemical cycle.
(A) Processive stepping pathway for KIF3A/B. Transitions k5A and k5B (dark red arrows) are associated with backsteps.
(B)Legend for the cycle in (A). KIF3A (green) and KIF3B (red) form the KIF3A/B dimer that moves on MTs (brown).
(C) Table of fit parameters and standard errors of fit for the global fit of the kinetic model to the data of Figures 2, S2A and S2B. Assignments of the mechanochemical transitions that correspond to each rate constant in the pathway are indicated. A three state model (combining states 3 and 4) was sufficient to model most of the data; however, fitting the model to the randomness data required four states for head B.
The data for head A were not sufficient to constrain parameter k4A, so in the actual fit, states 3A and 4A were lumped, equivalent to assuming a very rapid transition [3A] → [4A]. The lower bound for this transition was estimated using FitSpace. Similar model fits were carried out for kinesin-1 and kinesin-2 mutants (Tables S1, S3).