Figure 1.
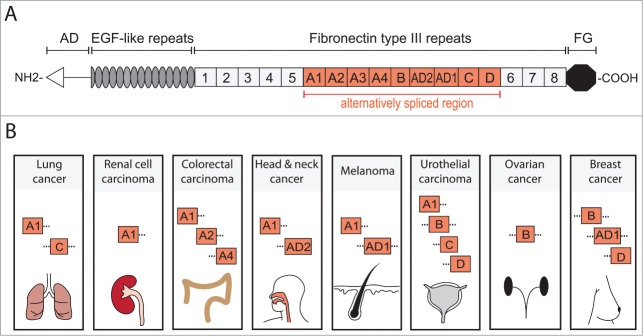
TNC structure and cancer associated domains. TNC is a multifunctional glycoprotein composed of several distinct domains. (A) Domain structure of full length human TNC protein (based on ref. 11). At the N-terminus, the assembly domain (AD) mediates the oligomerization of the protein where 2 trimers form a hexameric structure. Between the EGF-like repeats and the carboxy terminal fibrinogen globe (FG) are Fibronectin type III repeats (FNIII). In human TNC, 9 of the total 17 FNIII repeats are alternatively spliced providing the possibility of multiple different TNC isoforms. (B) Several alternatively spliced FNIII repeats have been identified in cancer. FNIII domains A1 and C are expressed in lung cancer and A1 domain in renal cell carcinoma.39,40,52 Colorectal carcinoma (CRC) expresses domains A1, A2 and A4 which are specifically enriched in CRC when compared to total TNC expression.51 Head and neck cancer exhibits A1 and AD2 domains while melanoma expresses A1 and AD1.53–56 In urothelial carcinoma, domains A1, B, C and D are present and associate with invasive cancer.57,58 FNIII domain B is expressed in ovarian cancer and is enriched compared to the short TNC isoform (lacking all alternatively spliced FNIII domains).59 Breast cancer expresses B and D domains that are associated with invasive behavior and the AD1 domain.55,60 It is important to note that these lists are not exhaustive and only include the domains that have been positively linked to a particular cancer. Information of complete isoforms is generally lacking. However, while the knowledge of different TNC isoforms expressed in cancer is still rudimentary and incomplete, the appearance of different domains in cancers may indicate a requirement for distinct aspects of TNC functions.
