Abstract
Aim:
Prolactin family hormones include growth hormone, placental lactogen and prolactin, which are able to regulate angiogenesis via NO and prostaglandins. However, their effects on vascular tone are not fully understood. The aim of this study was to evaluate the effects of prolactin family hormones on rat vascular tone in vitro.
Methods:
Aortic rings were prepared from adult male rats and precontracted with phenylephrine, then treated with the hormones and drugs. The tension was measured with isometric force displacement transducer connected to a polygraph. NO production and prostacyclin release in physiological solution was determined. Cultured rat aortic endothelial cells (RAECs) were treated with the hormones and drugs, and the phosphorylation of eNOS at serine 1177 was assessed using Western bolt analysis.
Results:
Administration of growth hormone or placental lactogen (0.01–100 nmol/L) induced endothelium-dependent vasodilation. Both the hormones significantly increased the phosphorylation of eNOS in RAECs and NO level in physiological solution. Preincubation with L-NAME blocked growth hormone- or placental lactogen-induced vasodilation and NO production. Preincubation with an antibody against growth hormone receptors blocked growth hormone- and placental lactogen-induced vasodilation. Addition of a single dose of prolactin (0.01 nmol/L) induced sustained vessel relaxation, whereas multiple doses of prolactin induced a biphasic contraction-relaxation effect. The vascular effects of prolactin depended on endothelium. Prolactin significantly increased the level of prostacyclin I2 in physiological solution. Preincubation with indomethacin or an antibody against prolactin receptors blocked prolactin-induced vasodilation.
Conclusion:
The prolactin family hormones regulate rat vascular tone, selectively promoting either relaxation or contraction of vascular smooth muscle via activation of either growth hormone receptors or prolactin receptors within the endothelium.
Keywords: endothelium, growth hormones, placental lactogen, prolactin, eNOS, NO, prostaglandins, aortic rings, aortic endothelial cells
Introduction
Endothelial cells produce a wide variety of vasoactive substances, such as nitric oxide (NO), prostaglandins (PGs) and growth factors, which regulate the following physiologic parameters: vascular tone, smooth muscle cell proliferation and angiogenesis1,2; however, an imbalance in both the production and the action of these factors may lead to “endothelial dysfunction”, promoting the vasoconstrictor responses associated with cardiovascular diseases3,4.
Cardiovascular physiology may be regulated by different groups of molecules, including hormones that promote either vasoconstriction or vasodilation, depending on their nature. For example, it is well known that angiotensin II (ANG II) induces a vasoconstrictor response mediated by L-type calcium channels5. In contrast, sex hormones such as 17-β-estradiol and progesterone induce vasodilation via the inhibition of the same L-type calcium channels6. The luteal hormone, relaxin, which is increased during pregnancy, also induces vasodilation, although this effect is mediated via increased NO production through the phosphorylation of endothelial NO synthase (eNOS)7. Among the reproductive hormones, the prolactin (PRL) family also exerts controversial vascular effects; the members of the PRL family include growth hormone (GH), placental lactogen (PL) and PRL, hormones that share structural and biological similarities8,9,10,11,12,13. GH and PRL are produced primarily in the pituitary gland but may also be locally produced by endothelial cells1,9,14. PL is produced by the placenta. The precise roles of the members of the PRL family in cardiovascular physiology, as well as their participation in the regulation of vascular tone, are not fully understood.
The evidence indicates that PRL, GH, and PL induce regional vasoconstriction via the inhibition of NO-mediated vasodilation, a process regulated by the β2-adrenergic receptors in coronary, renal and iliac vessels in anesthetized pigs15,16,17. Supporting these findings, another study has demonstrated that transgenic mice overexpressing the bovine GH gene exhibited increased mean arterial blood pressure, an effect associated with decreases in the lumens of the vascular beds of the hindquarters, which resulted in the development of hypertension18. Additionally, in vivo studies have suggested that low doses of PRL induce systemic pressor responses in the heart, whereas high doses of this same hormone induce systemic depressor activity in the heart19,20,21,22,23,24,25.
In contrast to the findings described above, both in vitro26 and in vivo studies27,28 indicate that GH induces eNOS-mediated NO production. In the human endothelial cell line, EAht926, high concentrations of GH regulate eNOS expression and NO production26, effects that also occur in human aortic endothelial cells29. Based on these findings, the loss of GH in humans decreases both blood flow and NO levels, whereas GH replacement restores these vascular responses27,30,31, indicating that patients with GH deficiency are at increased cardiovascular risk32.
These findings are indicative of the controversial roles played by PRL family members in the regulation of vascular function, roles that depend on the experimental models used, as well as the microenvironment surrounding the blood vessels, as each of these parameters may be key factors in the regulation of vascular responses9,33,34,35. Therefore, the aim of this study is to investigate the roles played by GH, PL, and PRL in the regulation of vascular tone, as well as the roles of the mediators involved in this process, using both isolated rat aortic rings and cultured aortic endothelial cells.
Materials and methods
Reagents
Recombinant human GH and PRL were each supplied by Bio Vision Research Products, and the PL utilized for this study was a rat recombinant standard obtained from the National Institutes of Health. Acetylcholine (ACh), indomethacin (INDO), NG-nitro-L-arginine methyl ester (L-NAME), phenylephrine (Phe) and salts for various physiologic solutions were purchased from Sigma Chemical Company (St Louis, MO, USA). Rat aortic endothelial cells were donated by Dr Guillermo CEBALLOS from the Instituto Politécnico Nacional, Mexico. Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) and penicillin/streptomycin, were purchased from Invitrogen (Carlsbad, CA, USA). The polyclonal anti-phosphoserine 1177 eNOS antibody and the monoclonal anti-eNOS antibody were each purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The monoclonal anti-PRL receptor (PRLR) antibody was purchased from Abcam (Cambridge, MA, USA), and the monoclonal anti-GH receptor (GHR) antibody was purchased from Novus Biological (Littleton, CO, USA). The horseradish peroxidase coupled secondary antibodies were obtained from Jackson Immune Research Laboratories (West, Grove, PA, USA); the Pierce ECL reagent and the western blotting substrate were purchased from Thermo Scientific (Rockford, IL, USA), and the rat prostaglandin I2 (PGI2) ELISA kit was purchased from MyBioSource (San Diego, CA, USA).
Tissue preparation
All animals were obtained from the Animal Facility of the School of Medicine of the Universidad Autonoma de San Luis Potosi according to the protocols approved by the Animal Care and Use Committee of the School of Medicine of the Universidad Autonoma de San Luis Potosi, Mexico and were handled in accordance with the standards set in the Eighth edition of the Guide for the Care and Use of Laboratory Animals. Adult male Wistar rats (250–350 g) were sacrificed via an overdose injection of sodium pentobarbital in accordance with our animal care protocols. Each of the experiments was performed as previously described36. Upon the sacrifice of each animal, the aorta was excised, cleansed of connective tissue and cut into 3-4 mm wide segments. The treatments were performed using individual aortic rings, both in the presence and the absence of the endothelium, which removed by gently rubbing the lumen with a cotton swab. The aortic rings were vertically fixed between two stainless steel hooks with a lower wire and immobilized at the bottom of an organ bath chamber containing 5 mL of physiologic solution (in mmol/L: 118 NaCl, 4.6 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.75 CaCl2, 20 HEPES and 10 glucose) kept at a pH of 7.4 and a temperature of 37 °C. The upper wire was connected to an isometric force displacement transducer (Grass FT03, Grass Instruments Co, Quincy, MA, USA), which was connected via amplifiers to a polygraph (Grass P122, Grass Instrument Co). Tension measurements were acquired in real time and analyzed using Polyview 2.0 software (ADI Instruments, Colorado Springs, CO, USA). A passive load of 2 g was applied, and the aortic segments were allowed to equilibrate for 60 min.
Vascular tone of rat aortic rings
The rat aortic vessels were subsequently precontracted with 2 μmol/L Phe37, followed by the administration of either increasing concentrations (0.01–100 nmol/L) or single concentrations (10 or 100 nmol/L) of GH or PL in both the presence and absence of the endothelium, and in the presence and absence of 100 μmol/L L-NAME (10 min pretreatment) and 10 μmol/L INDO (30 min pretreatment), a cyclooxygenase inhibitor38,39, or antibodies against GHR and PRLR (1 h pretreatment). For the PRL experiments, the aortic rings with and without endothelium were treated with either cumulative concentrations (0.01–100 nmol/L) or with single doses of the appropriate hormone (0.01 or 100 nmol/L), in either the presence or absence of Phe, 100 μmol/L L-NAME (10 min of pretreatment), 10 μmol/L INDO (30 min pretreatment), or antibodies against GHR and PRLR (1 h of pretreatment).
Regarding the procedures, the functionality of the endothelium was evaluated via the amount of relaxation induced by 10 μmol/L ACh. The effectiveness of the removal of the endothelium was confirmed by the absence of relaxation. Sustained relaxation (70% of precontracted tone) in response to ACh confirmed the presence of a functional endothelium40.
Western blot analysis
Cultured rat aortic endothelial cells were treated for 8 min with either GH or PL and homogenized in RIPA buffer; the protein concentration was determined via the bicinchoninic acid (BCA) method. Samples containing 30 μg of protein were loaded onto sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (4%–15%), run for 55 min at 200 V and transferred onto polyvinylidene fluoride membranes for 30 min at 100 V. Membranes were then blocked with 5% BSA and incubated overnight (4 °C) with the polyclonal anti-phospho serine 1177 eNOS primary antibody (1:200 dilution catalog sc-21871; Santa Cruz Biotechnology Inc). Membranes were washed and incubated with a secondary antibody (1:5000 dilution) for 2 h; protein was detected using chemiluminescence. The membranes were then stripped and incubated with a monoclonal anti eNOS antibody (1:200 dilution; catalog sc-8311, Santa Cruz Biotechnology Inc), and protein was detected via chemiluminescence. Images were analyzed using ImageJ software and normalized to β-actin.
Nitric oxide production
NO production was determined using the physiological solution that contained the aortic rings and the culture media in which the endothelial cells were kept by measuring both the total nitrites (NO2)/nitrates (NO3) concentration and the end products of NO metabolism, using the Griess reaction-based method36, following the treatments with GH, PL and PRL, in either the presence or the absence of L-NAME. Samples from the physiological assays were collected after 35 min, just after the end of the assay, and from the cell culture after 8 min of treatment. Briefly, 100 μL of samples were loaded into 96-well plates; 80 μL of 50 mmol/L VCl3 were added, followed by an additional 20 μL of Griess reagent; the samples were subsequently incubated for 30 min at 37 °C. Absorbance was determined at 540 nm, with a reference wavelength of 620 nm. The concentration of NO2 was calculated using a standard curve ranging from 1 to 200 μmol/L.
Prostacyclin production
The prostacyclin release experiments were performed independently of the tension experiments. Briefly, micro tubes containing rat aortic tissue and 250 μL of physiologic solution were placed into an incubated shaker and maintained at 37 °C and 160 rounds per minute (Innova 4000, New Brunswick, Canada) for 10 min to equilibrate before being treated with increasing concentrations of PRL (0.01–10 nmol/L) and with Phe (2 μmol/L) for 10 min. The tissue was then removed from the vial, and the supernatant was frozen in liquid nitrogen and stored at -80 °C until needed for further analysis. The aortic tissue was placed under a stereomicroscope along a millimeter ruler, and photographs were taken (∼90 pixels/mm resolution). The area of the aortic tissue was measured using ImageJ Software (National Institutes of Health, Bethesda, MD, USA) to normalize the PGI2 concentrations.
Supernatant samples were placed on an ELISA plate coated with anti-prostacyclin (PGI2) antibody (MyBioSource, San Diego, CA, USA). PGI2-horseradish peroxidase (HRP) conjugate was added and incubated at 37 °C for 1 h. HRP substrate was added and incubated at room temperature for 15 min. Stop solution was added; absorbance was determined at 450 nm with a Synergy HT microplate reader (Biotech Instruments, Winooski, VT, USA), and concentrations were obtained using a four-parameter logistic algorithm developed by Gen 5 Software (Biotech Instruments, Winooski, VT, USA).
Statistical analysis
The data were expressed as a mean±the standard errors of five independent experiments subjected to statistical analysis by either the Student́s t-test or the one-way ANOVA and the Tukey post hoc test for the detection of differences among groups. All statistical analyses were performed using GraphPad Prism 5 software. P values <0.05 were considered statistically significant.
Results
GH and PL induce vasodilation, whereas PRL induces dual effects in isolated rat aortic rings
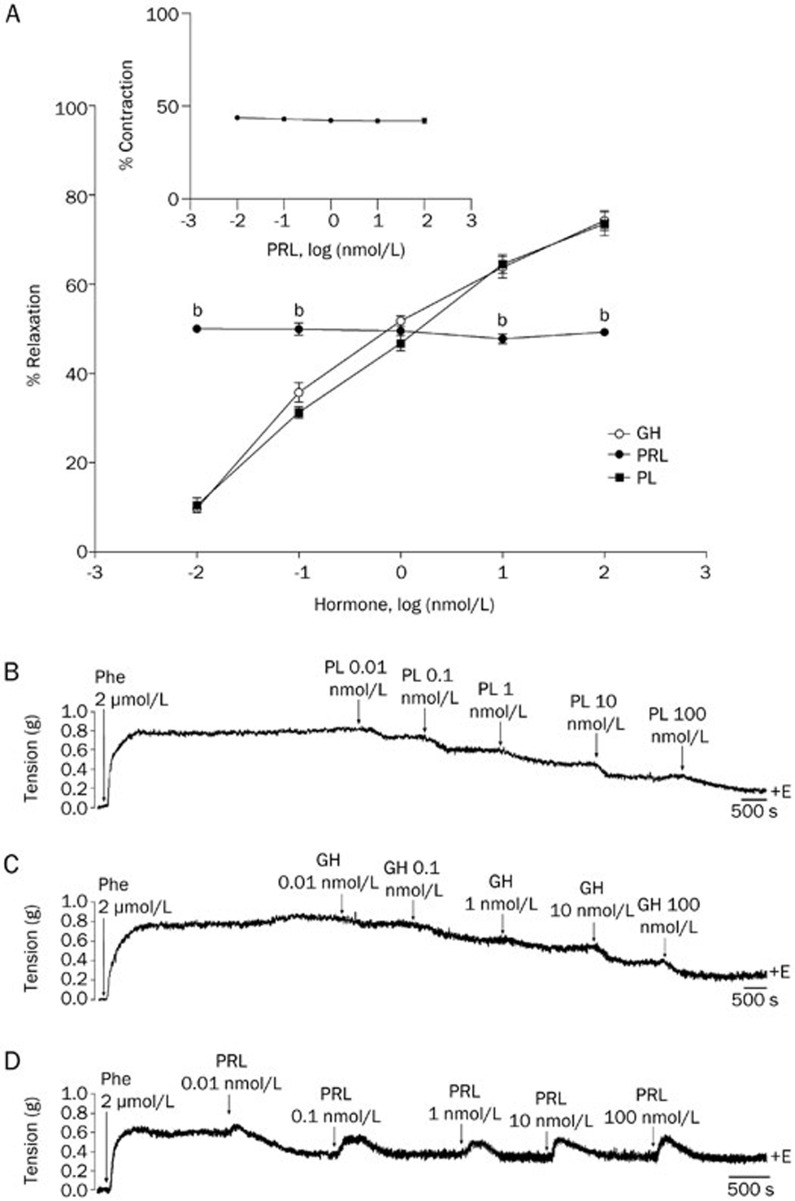
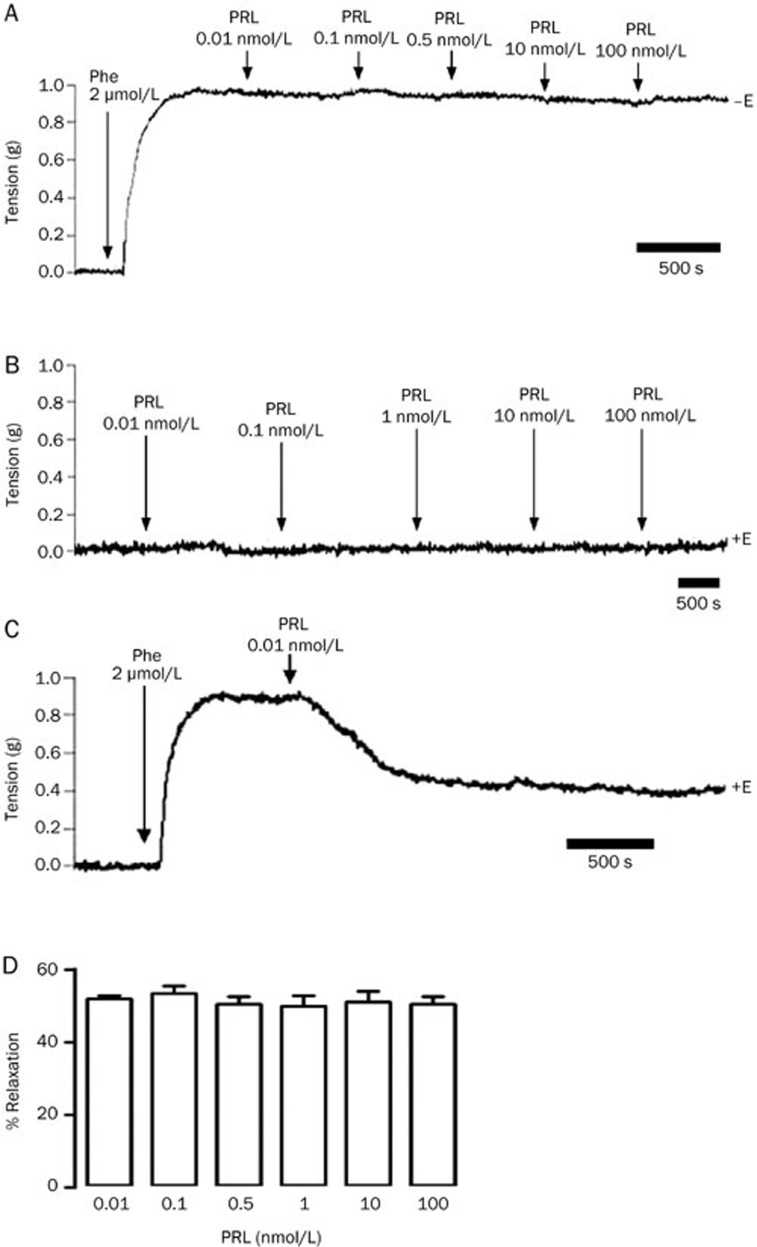
The direct actions of cumulative doses of GH, PL, and PRL were evaluated in Phe precontracted aortic rings. At the concentrations tested (0.01, 0.1, 1, 10, and 100 nmol/L), GH and PL induced comparable patterns of vasodilation in a cumulative dose-dependent patterns of 10%, 36%, 48%, 63%, and 73% and 10%, 30%, 46%, 63%, and 73%, respectively (Figure 1A–1C). However, PRL-induced vasodilation was more dose sensitive because the lowest concentration (0.01 nmol/L) promoted vasodilation comparable to 1 nmol/L GH/PL (50% vs 48%) (Figure 1A). Unlike GH and PL, which induced only vasodilation, the first dose of PRL exerted a vasodilator effect, whereas subsequent doses exerted a biphasic effect that consisted of a transient contractile effect of approximately 40% (insert Figure 1A), followed by a relaxation phase that was similar in magnitude to the contractions noted at each of the PRL concentrations tested (Figure 1D).
Figure 1.
Growth hormone (GH) and placental lactogen (PL), but not prolactin (PRL), induced relaxation in precontracted isolated rat aortic rings in a dose-dependent manner. Representative physiological recordings of tension, in grams (g), in response to 0.1–100 nmol/L of (B) GH, (C) PL, and (D) PRL. (A) Results are expressed as the percentages (%)±SEM of the relaxation [% of contraction in response to 2 μmol/L phenylephrine (Phe)] induced by GH, PL, and PRL. The insert at the top left represents the % of contraction induced by PRL during the transient contraction that occurred prior to the relaxation. +E, in the presence of the endothelium. n=5 animals in each group. bP<0.05 vs GH and PL.
GH and PL induce NO-mediated, endothelium-dependent vasodilation in isolated rat aortic rings
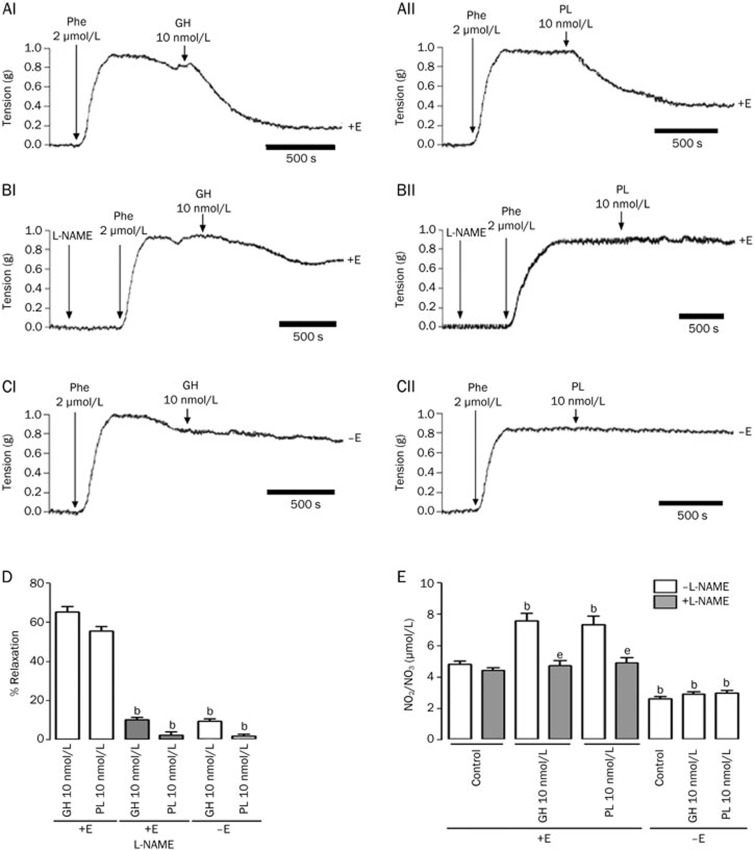
A single dose of either 10 nmol/L GH or PL induced vasodilatory effects of 65.3%, and 55.3%, respectively (Figures 2AI, 2AII, and 2D). The relaxation pattern was similar to the pattern of endothelium-dependent relaxation induced by ACh36,41. These effects were also dependent on NO production; in the case of the rings preincubated with L-NAME, the vasodilator effects were decreased to 10% for GH and 3% for PL (Figures 2BI, 2BII, and 2D). Furthermore, the relaxation induced by both hormones was absent when the endothelium was removed, as said parameter was only 9% for GH and 2% for PL (Figures 2CI, 2CII, and 2D). These physiological effects were directly associated with the production of NO because both GH and PL demonstrated increased NO production (7.5 and 7.1 μmol/L, respectively) compared with the control (4.6 μmol/L). NO production by both hormones was blocked by the presence of L-NAME (4.5 μmol/L for GH and 4.7 μmol/L for PL), and in the absence of the endothelium (2.8 μmol/L for both GH and PL) (Figure 2E). These effects were PG-independent because preincubation of the rings with INDO did not modify the vasodilation induced by the hormones (data not shown). These results indicate that the relaxation stimulated by both GH and PL was mediated by the endothelium via the release of NO.
Figure 2.
Growth hormone (GH) and placental lactogen (PL) induced endothelial- and nitric oxide-mediated relaxation in isolated rat aortic rings. Vessels were precontracted with 2 μmol/L phenylephrine (Phe). Once the Phe response was maintained, a single dose of either 10 nmol/L GH or PL was administered. Representative trace recordings of tension, in grams (g), in response to (AI) GH, (AII) PL +E, (BI) GH, (BII) PL after preincubation with 100 μmol/L L-NAME +E, (CI) GH and (CII) PL −E. NO production was calculated based on the accumulation of NO2 and NO3 in the physiologic solution, which contained the aortic rings, followed by the measurement of absorbance at 490 nm. The results are expressed as (D) the percentage of relaxation (% of contraction in response to phenylephrine) in the aortic rings and (E) the accumulation of NO2 and NO3 in the physiologic solution. Values are mean±SEM; bP<0.05 vs control or 10 nmol/L growth hormone (GH) or placental lactogen (PL) +E; eP<0.05 vs 10 nmol/L growth hormone (GH) or placental lactogen (PL) +E; n=5 animals in each group. +E, in the presence of the endothelium, −E, in the absence of the endothelium.
GH and PL induced vasodilation through their interaction with the GH receptor
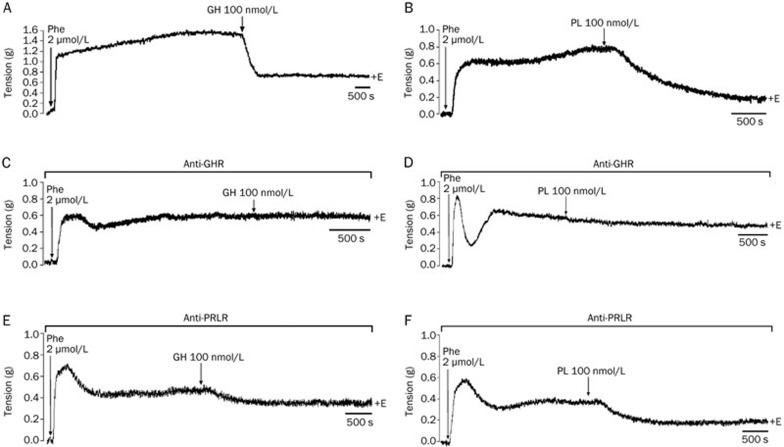
As GH and PL induced similar relaxation effects, we attempted to determine if they acted through the same receptor in the rat aorta. To determine which receptor was responsible for the hormonal differential effects, we used antibodies to block both the PRLR and the GHR. We found that preincubating the aortic rings with a monoclonal anti-GHR antibody for 1 h prior to the administration of the hormones blocked both GH- and PL-induced relaxation. Single doses of both 100 nmol/L GH and 100 nmol/L PL induced vasodilatory responses of 62% and 72%, respectively, whereas the relaxation in the presence of the antibody was 0% for GH and 12% for LP, demonstrating that these hormones exerted their relaxation effect via the GHR. Moreover, after preincubating the rings with a monoclonal antibody, anti-PRLR, GH, and PL continued to exert relaxation effects of 20.5% and 39.1%, respectively (Figure 3), suggesting that GH and PL induced relaxation in the rat aorta only thorough the GHR, as opposed to the PRLR. Preincubation with anti-GHR (Figure 3C and 3D) and anti-PRLR antibodies (Figure 3E and 3F) induced relaxations of approximately 52% and 47%, respectively, confirming the relaxation effects of the hormones.
Figure 3.
Antibodies against the growth hormone receptor (GHR), but not the prolactin receptor (PRLR), blocked the relaxation induced by growth hormone (GH) and placental lactogen (PL) in isolated rat aortic rings. Representative physiological recordings of tension, in grams (g), in rat aortic rings exposed to (A) 100 nmol/L GH, (B) 100 nmol/L PL, (C) 100 nmol/L GH in the presence of anti-GHR, (D) 100 nmol/L PL in the presence of anti-GHR antibodies, (E) 100 nmol/L GH in the presence of anti-PRLR antibodies and (F) 100 nmol/L PL in the presence of anti-PRLR antibodies. All experiments were performed in the presence of the endothelium (+E). n=2 animals in each group.
GH and PL induce eNOS phosphorylation at serine 1177 in rat aortic endothelial cells
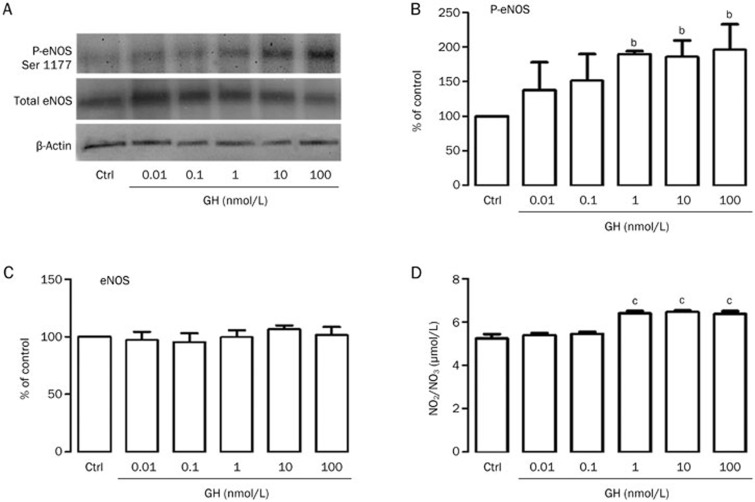
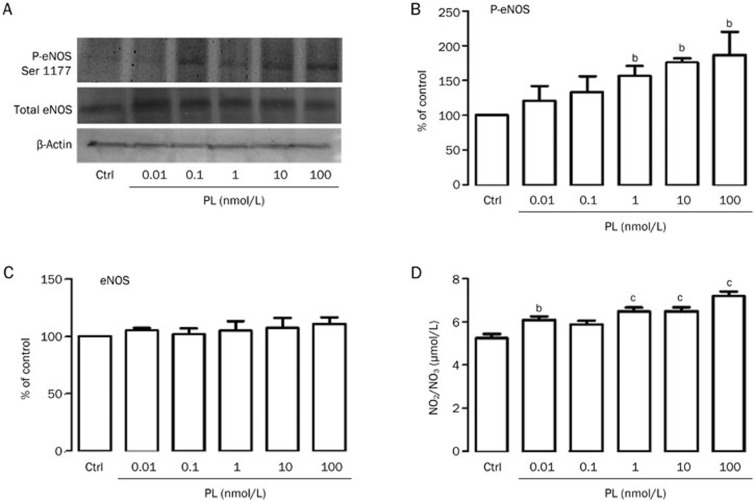
It is well known that vasoactive agents such as vascular endothelial growth factor (VEGF), bradykinin (BK) and ACh activate eNOS41,42,43,44,45 via the phosphorylation of the serine 1177 residue and the subsequent production of small amounts of NO44,46,47. We investigated whether GH and PL stimulate eNOS via the phosphorylation of its serine 1177 residue in cultured aortic endothelial cells. Under control conditions, a basal level of phosphorylation was detected. Treatments with increasing concentrations of GH and PL over 8 min increased the level of phosphorylation compared with the control (100%), as concentrations of 1, 10, and 100 nmol/L increased the level of phosphorylation by 89%, 85%, and 95% for GH (Figure 4A and 4B) and 56%, 75%, and 86% for PL (Figure 5A and 5B), without changing the total amount of eNOS protein (approximately 100%) (Figures 4C and 5C). The same hormonal concentrations that phosphorylated eNOS increased the production of NO. GH stimulated NO production as follows: 1 nmol/L GH produced 6.39 μmol/L NO; 10 nmol/L GH produced 6.45 μmol/L NO, and 100 nmol/L GH produced 6.38 μmol/L NO. PL stimulated the production of NO as follows: 1 nmol/L PL produced 6.47 μmol/L NO; 10 nmol/L PL produced 6.49 μmol/L NO, and 100 nmol/L PL produced 7.17 μmol/L NO compared with 5.2 μmol/L of the control (Figures 4D and 5D).
Figure 4.
Growth hormone (GH) induces both endothelial nitric oxide synthase (eNOS) serine 1177 phosphorylation and the release of nitric oxide (NO) in rat aortic endothelial cells (RAEC). Endothelial cells at 85% confluence were incubated for 8 min in either the presence or the absence of increasing concentrations of GH. Following the incubation period, the culture medium was collected, and the NO level was determined. (A) eNOS serine 1177 phosphorylation (p-eNOS Ser 1177) was analyzed via Western blot in RAEC. Cell lysates were separated via electrophoresis and transferred to membranes, followed by immunoblotting with antibodies directed against phospho-eNOS serine 1177, total eNOS and β-actin. (B) The quantification of eNOS serine 1177 phosphorylation was performed via densitometry after the compound was normalized to β-actin. (C) eNOS served as a control to confirm its constitutive presence in the cells following different treatments. (D) NO production was calculated based on the accumulation of NO2 and NO3 in the culture medium after 8 min of exposure to GH, followed by the measurement of its absorbance at 490 nm. The results are representative of 5 independent experiments. Values are mean±SEM. bP<0.05, cP<0.01 vs control (ctrl).
Figure 5.
Placental lactogen (PL) induces both endothelial nitric oxide synthase (eNOS) serine 1177 phosphorylation and the release of nitric oxide (NO) in rat aortic endothelial cells (RAEC). Endothelial cells at 85% confluence were incubated for 8 min in either the presence or the absence of increasing concentrations of PL. Following the incubation period, the culture medium was collected, and the NO level was determined. (A) eNOS 1177 phosphorylation (p-eNOS Ser 1177) was analyzed via Western blot in RAEC. Cell lysates were separated via electrophoresis and transferred to membranes, followed by immunoblotting with antibodies directed against phospho-eNOS serine 1177, total eNOS and β-actin. B) The quantification of eNOS serine 1177 phosphorylation was performed via densitometry after the compound was normalized to β-actin. (C) Total eNOS served as a control to confirm its constitutive presence in the cells after different treatments. (D) NO production was calculated based on the accumulation of NO2 and NO3 in the culture medium after 8 min of exposure in the presence of PL, followed by the measurement of absorbance at 490 nm. The results are representative of five independent experiments. Values are mean±SEM. bP<0.05 vs control (ctrl). cP<0.01 vs control (ctrl).
PRL's effects on the vascular tone of isolated rat aortic rings are both endothelium- and Phe-dependent
Even when the vascular effects induced by cumulative concentrations of PRL were different than those induced by GH and PL (Figure 1), each of the effects were endothelium-dependent, as the removal of the endothelium prevented PRL from exerting its effects (Figure 6A), and also prevented both GH and PL from exerting their effects (Figure 2CI and 2CII).
Figure 6.
Prolactin (PRL) induced endothelial- and phenylephrine (Phe)-mediated vascular effects in isolated rat aortic rings. Vessels were precontracted with 2 μmol/L Phe. Representative trace recordings of tension, in grams (g), in response to (A) cumulative (0.01–100 nmol/L) concentrations of PRL were administered in the presence of 2 μmol/L Phe −E; (B) cumulative (0.01–100 nmol/L) concentrations of PRL were administered in the absence of 2 μmol/L Phe 2 +E; (C) a single dose of 0.01 nmol/L PRL was administered in the presence of 2 μmol/L Phe +E; (D) the percentage of relaxation (% of contraction in response to Phe) in the aortic rings following the administration of single doses of PRL (0.01–100 nmol/L). Values are mean±SEM. n=5 animals in each group. Data that were not statically significant, bP<0.05. +E, in the presence of endothelium, −E, in the absence of endothelium.
To investigate the mediators of the effects exerted by PRL following the first dose, we hypothesized that pretreatment with Phe may be a rate-limiting step; therefore, we removed Phe from the physiologic solution and treated the aortic rings (with endothelium) with increasing concentrations of PRL. PRL did not induce any transient contractile effects at any of the concentrations tested (Figure 6B), suggesting that the presence of this catecholamine is necessary for PRL to exert its effects on the vasculature.
To determine whether the vasodilatory effect induced by the first dose of PRL was stable during the entire recording period and to confirm that the biphasic effects were not promoted by a single dose of PRL, the effects of which may be masked by the subsequent doses of the hormone, we used precontracted rings treated with a unique concentration of the hormone. We found that during a period of 35 min, the maximal level of relaxation was not modified, and no contractile effect was observed (Figure 6C). The same relaxation effect of approximately 50% was observed following the one-time administration of different doses of PRL (0.1–100 nmol/L) (Figure 6D).
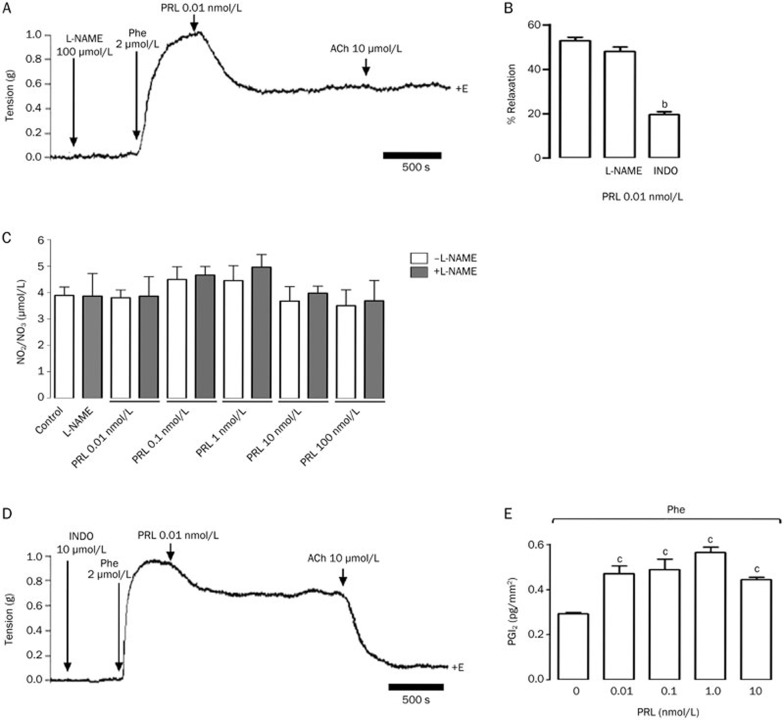
The PRL-induced dilatory effect is NO-independent but is also partially prostacyclin-dependent in isolated rat aortic rings
To identify the mediators of the vasodilatory effect of PRL, we used a pharmacological approach. We found that the vasodilation induced by PRL was NO-independent, because pretreatment with L-NAME did not block the relaxation effect induced by 0.01 nmol/L PRL (50% vs 46.5%) (Figure 7A and 7B). Furthermore, none of the PRL concentrations modified the basal production of NO in either the presence or the absence of L-NAME (control), confirming that this metabolite does not mediate the vascular effects induced by PRL (Figure 7C). We therefore hypothesized that PGs and other major vasodilatory agents48 may mediate the dilatory effect of the hormone. The aortic rings were preincubated for 30 min with INDO prior to precontraction with Phe, followed by the administration of 0.01 nmol/L of PRL. The administration of INDO decreased PRL-induced relaxation from 50% to 18% (Figure 7B and 7D); moreover, the increased production of prostacyclin I2(PGI2) by the aortic rings was detected at each of the PRL concentrations used in this study, beginning at 0.28 pg/mm3 in the control and increasing to 0.47, 0.48, 0.55, and 0.43 pg/mm3 at PRL concentrations of 0.01, 0.1, 1, and 10 nmol/L (Figure 7E), suggesting that the PRL-induced vasodilatory effect is mediated by both PGI2 and an unidentified mediator. This treatment did not affect the ACh response (Figure 7D) in the presence of the endothelium, as was reported previously41; moreover, preincubation with L-NAME did not modify the PRL-induced vasodilatory effect (Figure 7A and 7B) or the production of NO (Figure 7C) but did block ACh-induced NO-mediated endothelial relaxation (Figure 7A), confirming that PRL does not interfere with NO synthesis.
Figure 7.
Prolactin (PRL) induces prostacyclin (PG)-mediated relaxation and nitric oxide (NO)-independent relaxation in isolated rat aortic rings. The vessels were precontracted with 2 μmol/L phenylephrine (Phe). Representative trace recordings of tension, in grams (g), in response to (A) 0.01 nmol/L PRL after preincubation with 100 μmol/L L-NAME +E and (B) 0.01 nmol/L PRL after preincubation with 10 μmol/L indomethacin (INDO) +E; (C) NO production was calculated based on the quantification of NO2 and NO3 in a physiologic solution that bathed the aortic rings following treatment with PRL in the presence of L-NAME. (D) The percentage of relaxation (% of contraction in response to Phe) for each of the treatments; (E) the release of prostacyclin I2 (PGI2) (pg/mm2) in the physiologic solution, which contained the aortic rings, in the presence of Phe and in the presence of increasing concentrations of PRL (0.1–10 nmol/L). Values are mean±SEM. n=5 animals in each group. bP<0.05 vs 0.01 nmol/L prolactin. fP<0.01 vs control (PRL 0 nmol/L). +E, in the presence of endothelium.
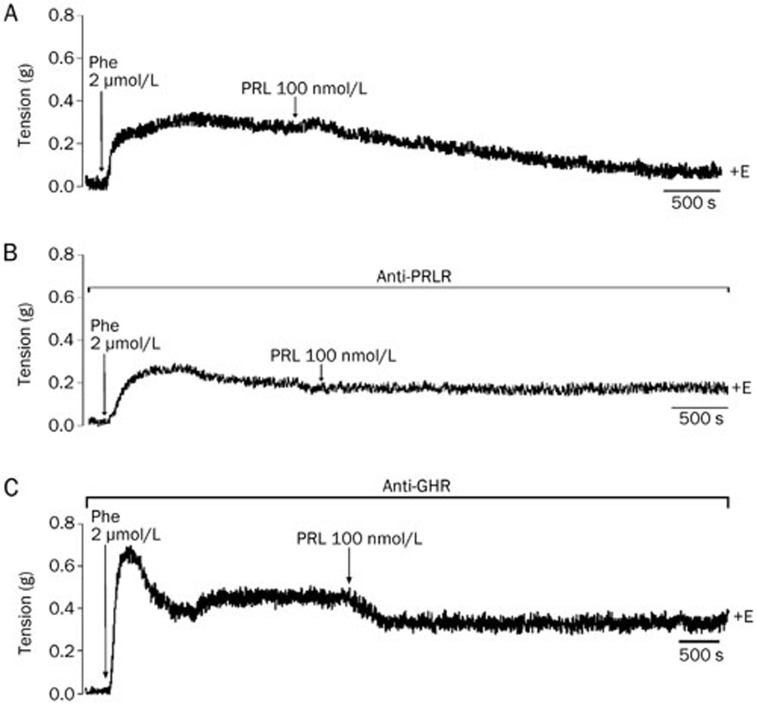
PRL induces its relaxation effect thorough its interaction with the PRLR
To determine which receptors are involved in PRL-induced vasodilation, we preincubated the aortic rings in both the absence (Figure 8A) and the presence of a monoclonal antibody against the PRLR for 1 h, prior to the incubation with the hormone. We found that preincubation with the antibody completely blocked PRL-induced vasodilation (Figure 8B). Moreover, preincubation with an anti-GHR antibody did not block PRL-induced vasodilation, because the hormone induced a 25% relaxation (Figure 8C). Once again, preincubation with anti-PRLR and anti-GHR antibodies induced relaxations in 48% and 44% of the rings, respectively (Figure 8B and 8C).
Figure 8.
Antibodies against the prolactin receptor (PRLR), but not the growth hormone receptor (GHR), blocked the relaxation induced by 100 nmol/L prolactin (PRL) in isolated rat aortic rings. Representative physiological recordings of tension, in grams (g), of rat aortic rings exposed to (A) 100 nmol/L PRL (B) in the presence of an anti-PRLR antibody and (C) an anti-GHR antibody. All experiments were performed in the presence of endothelium (+E). n=2 animals in each group.
Discussion
In the present study, we demonstrated that GH and PL induce vasodilation in an isolated rat aortic ring model in a cumulative dose-dependent manner, an effect that results from the endothelial release of NO, like the effect induced by ACh41,49. In contrast, PRL did not induce these effects, as the first dose of PRL induced a vasodilatory response of approximately 50% of the maximum contraction, but subsequent doses exerted a biphasic effect that consisted primarily of contraction followed by relaxation.
The relaxation induced by each member of the PRL family was endothelium-dependent; when the endothelium was removed, no effects were observed, indicating that the hormones act directly on endothelial cells.
GH and PL, but not PRL, induced NO-dependent relaxation; the effects induced by both hormones were completely blocked by L-NAME, an inhibitor of NOS41. The production of endothelial derived NO results from the calcium-calmodulin activation of eNOS via phosphatidylinositol 3-kinase, followed by the phosphorylation of eNOS at its serine1177 residue44,50,51. The diffusion of NO into smooth muscle cells activates soluble guanylyl cyclase, which increases intracellular cGMP levels and activates protein kinase G1, which results in the phosphorylation of the inositol-triphosphate receptor-associated cGMP kinase substrate and the sarcoplasmic reticulum ATPase; this decreases intracellular calcium flux and promotes vascular relaxation50,52. In aortic endothelial cells, we observed a basal level of eNOS phosphorylation at the serine 1177 residue under control conditions, which was enhanced in the presence of GH and PL as reported previously26,29. The GH- and PL-induced increases in eNOS phosphorylation occurred after 8 min of treatment, which was the approximate time in which both hormones exerted their NO-dependent vasodilatory effects.
A collection of evidence suggests that GH directly contributes to the regulation of vascular growth and function, and GH receptors have been identified within different vascular beds1,26,53. Clinical studies indicate that GH is one of many factors regulating vascular homeostasis1,53; Napoli et al28 demonstrated that the exogenous administration of GH induced local vasodilation in a rat model, an effect that was related to increased of NO levels and promoted endothelial sensitivity to vasorelaxants, such as ACh; however, they did not clarify which signaling molecules were involved but hypothesized that the local action of the hormone was responsible for the observed effects. We demonstrated that GH and PL act directly on the GHR in endothelial cells, promoting both vasodilation and NO release. Although the effects induced by PL on the vasculature are not fully understood, our data are among the first to suggest the role played by this hormone in the endothelium and in the NO-dependent relaxation of the aorta, a process that may be related to the redistribution of blood flow that occurs during pregnancy7,54. It is important to note that the experimental approaches utilized, as well as the conditions under which the experiments were undertaken, are important factors to consider, because the vasoconstrictor effects induced by GH and PL via the inhibition of NO have been reported in other vascular beds and in the setting of other experimental approaches15,16,18, findings suggestive of the differential actions of these hormones in different vascular beds. One possible explanation for these differential effects is the presence of the GHR in the endothelium. It was reported previously that the rat aorta expresses the GHR55; we confirmed its presence in the aortic rings, and GH and PL induced their relaxation effects via their interaction with this receptor; blocking the receptor with an anti-GHR antibody blocked these hormone-induced relaxation effects.
By contrast, because of a single dose of PRL induced NO-independent and endothelial-dependent vasodilation, we concluded that PGs may be one of the endothelial mediators of this effect, as it has been proven that PGs induce the relaxation of aortic smooth muscle. When we incubated the isolated rat aortic rings (with endothelium) in the presence of INDO prior to the administration of Phe and PRL, the PRL-induced vasodilatory effect was partially blocked, suggesting a role for endothelial PGs in the PRL-induced vascular responses in this model. We found that PRL induced the production of PGI2, an endothelium-derived vasodilatory agent56, which appears to be one of the primary factors that contributes to the vasodilatory effect induced by this hormone, findings that support the above hypothesis. However, the possibility that another arachidonic acid derivative may be involved in the local modulation of vascular effects is reasonable, because the vasodilation was only partially blocked by INDO, suggesting that this effect may also be mediated by other dilatory PGs, which may regulate the homeostasis of the cardiovascular system in conjunction with PGI256,57; however, the possibility that other types of molecules may also be associated with PRL-induced vasodilation cannot be discounted. Each of these effects was mediated via the interaction of PRL with the PRLR, as was demonstrated via the complete inhibition of the vasodilatory effect in the presence of an antibody that blocked the PRLR. Moreover, it was reported previously that the aortic endothelium expresses both the short and the long forms of the PRLR58.
It is worth mentioning that the vascular effects induced by PRL did not affect ACh-induced vasodilation as was reported previously41. In that study, the vasodilatory effect exerted by increasing concentrations of ACh was not modified by a single dose of PRL in isolated rat aortic rings. However, those experiments were undertaken in the presence of INDO, which may have masked the vasodilatory response induced by the first dose of PRL.
On the other hand, successive doses of PRL precipitated a biphasic effect. It has been reported that different molecules59,60 including hormones61, exert dual effects comparable with the effects exerted by PRL; for example, angiotensin II causes transient coronary constriction and exerts positive inotropic effects in some cardiovascular models62,63 as a result of either progressive endothelial angiotensin I receptor internalization or receptor desensitization64, effects associated with tachyphylaxis. In our study, both the effects of the successive doses of PRL and the decreased vasodilation induced by the first PRL dose may be related to an endothelial desensitization process, as cumulative doses of PRL did not exert direct effects on aortic smooth muscle. Castillo-Hernández et al61 also demonstrated that the desensitization induced by angiotensin II is dependent on the coronary endothelial luminal membrane in a model of isolated heart preparations. Regarding the contractile effect of PRL, Molinari et al17 previously reported that the administration of PRL induced vasoconstriction in anesthetized pigs, an effect mediated via ß2-adrenergic receptors that was related to both decreased NO production and the phosphorylation of ERK, p38 and Akt in porcine aortic endothelial cells. In this study, we found that PRL did not induce increases in NO production compared with the control; however, we found that successive doses of PRL induced the transient contraction of the vessel, followed by a relaxation. In the case of the methods used by Molinari et al, PRL was administered to anesthetized pigs in which endogenous PRL was already circulating in the bloodstream; the exogenous administration of PRL to these animals exerted effects similar to those observed with successive administrations of PRL. They observed a contractile effect but not a relaxing effect, because they could not administer a “first” dose of PRL to the animals that already maintained a constant concentration of endogenous hormone in their bloodstream.
Regarding the effects of β2-adrenergic receptors, Molinari et al found that PRL significantly reduced the regional vasodilation caused by β2-adrenergic agents and suggested that the hormone caused coronary, mesenteric, renal, and iliac vasoconstriction by blocking a tonic vasodilatory effect mediated by β2-adrenergic receptors. These findings suggest that the contractile effect of PRL blocks the relaxation effect exerted following the stimulation of β2-adrenergic receptors, not that PRL exerts its effect by interacting with those receptors. The findings of this study are not in conflict with the results of Molinari' study, as this study explored a different mechanism of the same effect, a mechanism that may be mediated by multiple factors. These data suggest that PRL may be associated with an endothelial mediator that modifies the degree of tension following successive hormone doses. In accordance with this findings, it was recently reported that the chronic administration of PRL induces an increased response to contractile agents (KCl and ACh) in the smooth muscle cells of the seminal vesicles; on the other hand, the inhibition of PRL with bromocriptine decreased this contractile response65, suggesting that circulating PRL may regulate smooth muscle contraction and vascular tone, as observed in this study.
The effects exerted by the members of the PRL family may be explained in part by the structural similarities of the hormones, as there is an 85% sequence similarity between the peptide sequences of hGH and hPL, whereas hPRL shares approximately 25% of its sequences with the other hormones. Additionally, hPRL has a very weak affinity for the GH receptor, whereas GH, PL, and PRL each bind with high affinity to the PRL receptor9; moreover, we demonstrated that both GH and PL induced their relaxation effects via the GHR and PRLR, respectively. In addition to these findings, we observed that following Phe-induced contraction, a relaxation effect of approximately 50% occurred, an effect that stabilized after 50 min, in the aortic rings that were preincubated with either anti-GHR or PRLR antibodies. Following the addition of GH, PL, or PRL, these hormones exerted their relaxation effects in a lower magnitude than in the absence of the antibodies. These effects may because the antibodies used in this study bind to hormone receptors and act as hormone receptor agonists; therefore, because the hormones induce relaxation effects, the antibodies that either stimulate or bind to their receptors may exert the same effects. Consequently, because the aortic rings were already relaxed, the vasodilation induced by the hormones would not be of the same magnitude, but it would still be present. Even during instances in which GH and PL induced a partial relaxation in the presence of an anti-GHR antibody, it's possible that these effects were attributed to the antibodies as opposed to the participation of multiple receptors, as incubation with the anti-GHR antibody completely blocked the relaxation effects exerted GH and PL, and incubation with an anti-PRLR antibody completely blocked the relaxation induced by PRL, confirming that PRL acts via a different receptor than both GH and PL.
The results of the present study suggest that GH and PL share a common mechanism by which they induce vasodilation, a mechanism that is not shared by PRL. Although a single dose of this hormone induced vasodilation, this effect was not dependent on the production of NO, which was not the case for both GH and PL.
The potential effects exerted by GH, PL, and PRL on the macro-vasculature were implicated via the control of central aortic pressures, which resulted in the modulation of several parameters that affect the cardiovascular system, including heart rate, stroke volume, arterial stiffness and peripheral arterial resistance66. Dysfunction due to a hormonal imbalance may determine both the development and the progression of cardiovascular diseases, such as hypertension, pre-eclampsia, coronary ischemia, diabetes, aging, and hypercholesterolemia. Therefore, further study is necessary to understand the precise mechanisms of that underlie these functional effects.
In summary, these data describe the selective effects exerted by the members of the PRL family on vascular tone. GH, PL, and PRL each induced endothelium-dependent vasodilation. The effects of GH and PL were NO-dependent and mediated via interaction with the GHR, whereas the effects of PRL were partially PGI2-dependent and were mediated by the PRLR. Additionally, multiple doses of PRL induced a biphasic contraction-relaxation effect. The mechanisms and signaling molecules involved in these processes warrant further investigation under both normal and altered vascular conditions.
Author contribution
Carmen GONZALEZ designed and directed the study and wrote the manuscript. Hector ROSAS-HERNANDEZ performed the study, collected data and collaborated with the writing of the manuscript. Brenda JURADO-MANZANO collected data. Manuel Alejandro RAMIREZ-LEE collected data, designed the graphics and collaborated with the writing of the manuscript. Samuel SALAZAR-GARCIA collected data. Pedro Pablo MARTINEZ-CUEVAS collected data. Aída Jimena VELARDE-SALCEDO collected data. Humberto MORALES-LOREDO collected data. Ricardo ESPINOSA-TANGUMA collected data and collaborated with the writing of the manuscript. Syed F ALI provided important reagents and reviewed the language of the manuscript. Rafael RUBIO provided important reagents and reviewed the language of the manuscript.
Acknowledgments
This study was supported by the Consejo Nacional de Ciencia y Tecnología CONACYT-Mexico (84576 and 134595) and the Academic and Research Support Program (PROMEP-103.5/08/5637, C08-FAI-10-13.49, CO8-PIFI-05-4.4, and C14-PIFI-08-06.06), and was also supported in part by an appointment to the Research Participation Program at the National Center for Toxicological Research, administered by the Oak Ridge Institute for Science and Education, through an interagency agreement between the US Department of Energy and the US Food and Drug Administration. Hector ROSAS-HERNANDEZ would like to thank PROMEP (PROMEP/103.5/07/2574) and CONACyT (317768) for the fellowships that each provided; Brenda JURADO-MANZANO, Samuel SALAZAR-GARCIA and Manuel Alejandro RAMIREZ-LEE would like to thank CONACyT for the No 55849; 278347 and 257942 fellowships.
References
- Clapp C, Thebault S, Jeziorski MC, Martinez De La Escalera G. Peptide hormone regulation of angiogenesis. Physiol Rev. 2009;89:1177–215. doi: 10.1152/physrev.00024.2009. [DOI] [PubMed] [Google Scholar]
- Tirapelli CR, Bonaventura D, Tirapelli LF, de Oliveira AM. Mechanisms underlying the vascular actions of endothelin 1, angiotensin II and bradykinin in the rat carotid. Pharmacology. 2009;84:111–26. doi: 10.1159/000231974. [DOI] [PubMed] [Google Scholar]
- Cotton JM, Kearney MT, Shah AM. Nitric oxide and myocardial function in heart failure: friend or foe. Heart. 2002;88:564–6. doi: 10.1136/heart.88.6.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckles SP, Miller VM. Hormonal modulation of endothelial NO production. Pflugers Arch. 2010;459:841–51. doi: 10.1007/s00424-010-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KH, Kim MS, Kim JH, Rhim BY, Lee WS, Kim CD, et al. Angiotensin II-induced aortic ring constriction is mediated by phosphatidylinositol 3-kinase/L-type calcium channel signaling pathway. Exp Mol Med. 2009;41:569–76. doi: 10.3858/emm.2009.41.8.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairrao E, Alvarez E, Carvas JM, Santos-Silva AJ, Verde T. Non-genomic vasorelaxant effects of 17β-estradiol and progesterone in rat aorta are mediated by L-type Ca2+ current inhibition. Acta Pharmacol Sin. 2012;33:615–24. doi: 10.1038/aps.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP. Maternal vasodilation in pregnancy: the emerging role of relaxin. Am J Physiol Regul Integr Comp Physiol. 2011;301:R267–75. doi: 10.1152/ajpregu.00156.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19:225–68. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- Corbacho AM, Martinez De La Escalera G, Clapp C. Roles of prolactin and related members of the prolactin/growth hormone/placental lactogen family in angiogenesis. J Endocrinol. 2002;173:219–38. doi: 10.1677/joe.0.1730219. [DOI] [PubMed] [Google Scholar]
- Ho KK, O'Sullivan AJ, Hoffman DM. Metabolic actions of growth hormone in man. Endocr J. 1996;43:S57–63. doi: 10.1507/endocrj.43.suppl_s57. [DOI] [PubMed] [Google Scholar]
- Lewis UJ. Growth Hormone What is it and what does it do. Trends Endocrinol Metab. 1992;3:117–21. doi: 10.1016/1043-2760(92)90099-m. [DOI] [PubMed] [Google Scholar]
- Nicoll CS, Mayer GL, Russell SM. Structural features of prolactins and growth-hormones that can be related to their biological properties. Endocr Rev. 1986;7:169–203. doi: 10.1210/edrv-7-2-169. [DOI] [PubMed] [Google Scholar]
- Walker WH, Fitzpatrick SL, Barrera-Saldana HA, Resendez-Perez D, Saunders GF. The human placental lactogen genes: structure, function, evolution and transcriptional regulation. Endocr Rev. 1991;12:316–28. doi: 10.1210/edrv-12-4-316. [DOI] [PubMed] [Google Scholar]
- Clapp C, Martinez de la Escalera L, Martinez de la Escalera G. Prolactin and blood vessels: a comparative endocrinology perspective. Gen Comp Endocrinol. 2012;176:336–40. doi: 10.1016/j.ygcen.2011.12.033. [DOI] [PubMed] [Google Scholar]
- Grossini E, Molinari C, Battaglia A, Mary DA, Ribichini F, Surico N, et al. Human placental lactogen decreases regional blood flow in anesthetized pigs. J Vasc Res. 2006;43:205–13. doi: 10.1159/000090950. [DOI] [PubMed] [Google Scholar]
- Molinari C, Battaglia A, Bona G, Grossini E, Mary DA, Vacca G. The role of nitric oxide in the coronary vasoconstriction caused by growth hormone in anaesthetized pigs. Exp Physiol. 2000;85:203–8. [PubMed] [Google Scholar]
- Molinari C, Grossini E, Mary DA, Uberti F, Ghigo E, Ribichini F, et al. Prolactin induces regional vasoconstriction through the beta2-adrenergic and nitric oxide mechanisms. Endocrinology. 2007;148:4080–90. doi: 10.1210/en.2006-1577. [DOI] [PubMed] [Google Scholar]
- Bohlooly YM, Carlson L, Olsson B, Gustafsson H, Andersson IJ, Törnell J, et al. Vascular function and blood pressure in GH transgenic mice. Endocrinology. 2001;142:3317–23. doi: 10.1210/endo.142.8.8296. [DOI] [PubMed] [Google Scholar]
- Karmazyn M, Daly MJ, Moffat MP, Dhalla NS. A possible mechanism of inotropic action of prolactin on rat heart. Am J Physiol. 1982;243:E458–63. doi: 10.1152/ajpendo.1982.243.6.E458. [DOI] [PubMed] [Google Scholar]
- Manku MS, Nassar BA, Horrobin DF. Effects of prolactin on the responses of rat aortic and arteriolar smooth-muscle preparations to noradrenaline and angiotensin. Lancet. 1973;302:991–4. doi: 10.1016/s0140-6736(73)91089-1. [DOI] [PubMed] [Google Scholar]
- Manku MS, Horrobin DF, Karmazyn M, Cunnane SC. Prolactin and zinc effects on rat vascular reactivity: possible relationship to dihomo-gamma-linolenic acid and to prostaglandin synthesis. Endocrinology. 1979;104:774–9. doi: 10.1210/endo-104-3-774. [DOI] [PubMed] [Google Scholar]
- Nassar BA, Manku MS, Reed JD, Tynan M, Horrobin DF. Actions of prolactin and frusemide on heart rate and rhythm. Br Med J. 1974;2:27–9. doi: 10.1136/bmj.2.5909.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills DE, Buckman MT, Peake GT. Effect of prolactin administration and suppression on blood pressure and body fluid compartments in the rat. Endocrinology. 1981;109:1590–6. doi: 10.1210/endo-109-5-1590. [DOI] [PubMed] [Google Scholar]
- Mills DE, Ward RP. Effect of prolactin on blood pressure and cardiovascular responsiveness in the rat. Proc Soc Exp Biol Med. 1986;181:3–8. doi: 10.3181/00379727-181-42217. [DOI] [PubMed] [Google Scholar]
- Mtabaji JP, Manku MS, Horrobin DF. Vascular actions of furosemide and bumetanide on the rat superior mesenteric vascular bed: interactions with prolactin and prostaglandins. Can J Physiol Pharmacol. 1976;54:357–66. doi: 10.1139/y76-050. [DOI] [PubMed] [Google Scholar]
- Thum T, Tsikas D, Frolich JC, Borlak E. Growth hormone induces eNOS expression and nitric oxide release in a cultured human endothelial cell line. FEBS Lett. 2003;555:567–71. doi: 10.1016/s0014-5793(03)01356-5. [DOI] [PubMed] [Google Scholar]
- Boger RH, Skamira C, Bode-Boger SM, Brabant G, von zur Muhlen A, Frolich JC. Nitric oxide may mediate the hemodynamic effects of recombinant growth hormone in patients with acquired growth hormone deficiency. A double-blind, placebo-controlled study. J Clin Invest. 1996;98:2706–13. doi: 10.1172/JCI119095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli R, Guardasole V, Angelini V, D'Amico F, Zarra E, Matarazzo M, et al. Acute effects of growth hormone on vascular function in human subjects. J Clin Endocrinol Metab. 2003;88:2817–20. doi: 10.1210/jc.2003-030144. [DOI] [PubMed] [Google Scholar]
- Li G, Del Rincon JP, Jahn LA, Wu Y, Gaylinn B, Thorner MO, et al. Growth hormone exerts acute vascular effects independent of systemic or muscle insulin-like growth factor I. J Clin Endocrinol Metab. 2008;93:1379–85. doi: 10.1210/jc.2007-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola M, Bonadonna S, Doga M, Giustina A. Clinical review: Growth hormone and cardiovascular risk factors. J Clin Endocrinol Metab. 2005;90:1864–70. doi: 10.1210/jc.2004-0545. [DOI] [PubMed] [Google Scholar]
- Capaldo B, Guardasole V, Pardo F, Matarazzo M, Di Rella F, Numis F, et al. Abnormal vascular reactivity in growth hormone deficiency. Circulation. 2001;103:520–4. doi: 10.1161/01.cir.103.4.520. [DOI] [PubMed] [Google Scholar]
- Napoli R, Guardasole V, Matarazzo M, Palmieri EA, Oliviero U, Fazio S, et al. Growth hormone corrects vascular dysfunction in patients with chronic heart failure. J Am Coll Cardiol. 2002;39:90–5. doi: 10.1016/s0735-1097(01)01707-7. [DOI] [PubMed] [Google Scholar]
- McMurtry JP, Wexler BC. Hyperprolactinemia and hyperadrenocorticism accompanied by normal blood pressure in Sprague-Dawley rats. Proc Soc Exp Biol Med. 1981;168:114–8. doi: 10.3181/00379727-168-41243. [DOI] [PubMed] [Google Scholar]
- Nassar BA, Horrobin DF, Tynan M, Manku MS, Davies PA. Seasonal and sexual variations in the responsiveness of rabbit hearts to prolactin. Endocrinology. 1975;97:1008–13. doi: 10.1210/endo-97-4-1008. [DOI] [PubMed] [Google Scholar]
- Wessels F, Hoffmann D, Wagner H, Zumkley H. Influence of family history of hypertension on the relationships between blood pressure, body weight, electrolyte metabolism, renin, prolactin and parathormone. Clin Sci (Lond) 1981;61:359s–62s. doi: 10.1042/cs061359s. [DOI] [PubMed] [Google Scholar]
- Rosas-Hernandez H, Jimenez-Badillo S, Martinez-Cuevas PP, Gracia-Espino E, Terrones H, Terrones M, et al. Effects of 45-nm silver nanoparticles on coronary endothelial cells and isolated rat aortic rings. Toxicol Lett. 2009;191:305–13. doi: 10.1016/j.toxlet.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–63. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- Engler MB, Engler MM, Browne A, Sun YP, Sievers R. Mechanisms of vasorelaxation induced by eicosapentaenoic acid (20:5n-3) in WKY rat aorta. Br J Pharmacol. 2000;131:1793–9. doi: 10.1038/sj.bjp.0703754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue YL, Shi HX, Murad F, Bian K. Vasodilatory effects of cinnamaldehyde and its mechanism of action in the rat aorta. Vasc Health Risk Manag. 2011;7:273–80. doi: 10.2147/VHRM.S15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurpinar T, Gok S. Vasodilator effects of cromakalim and HA 1077 in diabetic rat aorta. Swiss Med Wkly. 2012;142:w13558. doi: 10.4414/smw.2012.13558. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Corbacho AM, Eiserich JP, Garcia C, Lopez-Barrera F, Morales-Tlalpan V, et al. 16K-prolactin inhibits activation of endothelial nitric oxide synthase, intracellular calcium mobilization, and endothelium-dependent vasorelaxation. Endocrinology. 2004;145:5714–22. doi: 10.1210/en.2004-0647. [DOI] [PubMed] [Google Scholar]
- Aschner JL, Zeng H, Kaplowitz MR, Zhang Y, Slaughter JC, Fike CD. Heat shock protein 90-eNOS interactions mature with postnatal age in the pulmonary circulation of the piglet. Am J Physiol Lung Cell Mol Physiol. 2009;296:L555–64. doi: 10.1152/ajplung.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rosa AP, Montoya AB, Martinez-Cuevas P, Hernández-Ledesma B, León-Galván MF, De León-Rodríguez A, et al. Tryptic amaranth glutelin digests induce endothelial nitric oxide production through inhibition of ACE: antihypertensive role of amaranth peptides. Nitric Oxide. 2010;23:106–11. doi: 10.1016/j.niox.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Garcia C, Aranda J, Arnold E, Thébault S, Macotela Y, López-Casillas F, et al. Vasoinhibins prevent retinal vasopermeability associated with diabetic retinopathy in rats via protein phosphatase 2A-dependent eNOS inactivation. J Clin Invest. 2008;118:2291–300. doi: 10.1172/JCI34508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema VJ, Marrero MB, Venema RC. Bradykinin-stimulated protein tyrosine phosphorylation promotes endothelial nitric oxide synthase translocation to the cytoskeleton. Biochem Biophys Res Commun. 1996;226:703–10. doi: 10.1006/bbrc.1996.1417. [DOI] [PubMed] [Google Scholar]
- Bird IM. Endothelial nitric oxide synthase activation and nitric oxide function: new light through old windows. J Endocrinol. 2011;210:239–41. doi: 10.1530/JOE-11-0216. [DOI] [PubMed] [Google Scholar]
- Rafikov R, Fonseca FV, Kumar S, Pardo D, Darragh C, Elms S, et al. eNOS activation and NO function: structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity. J Endocrinol. 2011;210:271–84. doi: 10.1530/JOE-11-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte PM. Endothelium-dependent contractions in hypertension: when prostacyclin becomes ugly. Hypertension. 2011;57:526–31. doi: 10.1161/HYPERTENSIONAHA.110.165100. [DOI] [PubMed] [Google Scholar]
- Sausbier M, Schubert R, Voigt V, Hirneiss C, Pfeifer A, Korth M, et al. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ Res. 2000;87:825–30. doi: 10.1161/01.res.87.9.825. [DOI] [PubMed] [Google Scholar]
- Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol. 2001;280:F193–206. doi: 10.1152/ajprenal.2001.280.2.F193. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Mendelsohn ME. Synergistic activation of endothelial nitric-oxide synthase (eNOS) by HSP90 and Akt: calcium-independent eNOS activation involves formation of an HSP90-Akt-CaM-bound eNOS complex. J Biol Chem. 2003;278:30821–7. doi: 10.1074/jbc.M304471200. [DOI] [PubMed] [Google Scholar]
- Walford G, Loscalzo J. Nitric oxide in vascular biology. J Thromb Haemost. 2003;1:2112–8. doi: 10.1046/j.1538-7836.2003.00345.x. [DOI] [PubMed] [Google Scholar]
- Thum T, Bauersachs J.Growth hormone regulates vascular function - what we know from bench and bedside Eur J Clin Pharmacol 20066229–32.16341856 [Google Scholar]
- Krause BJ, Hanson MA, Casanello P. Role of nitric oxide in placental vascular development and function. Placenta. 2011;32:797–805. doi: 10.1016/j.placenta.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickman A, Friberg P, Adams MA, Matejka GK, Brantsing C, Guron G, et al. Induction of growth hormone receptor and insuline-like growth factor-1 mRNA in aorta and caval vein during hemodynamic challenge. Hypertension. 1997;29:123–30. doi: 10.1161/01.hyp.29.1.123. [DOI] [PubMed] [Google Scholar]
- Kawabe J, Ushikubi F, Hasebe N. Prostacyclin in vascular diseases. Recent insights and future perspectives. Circ J. 2010;74:836–43. doi: 10.1253/circj.cj-10-0195. [DOI] [PubMed] [Google Scholar]
- Blanco-Rivero J, Cachofeiro V, Lahera V, Aras-Lopez R, Márquez-Rodas I, Salaices M, et al. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hypertension. 2005;46:107–12. doi: 10.1161/01.HYP.0000171479.36880.17. [DOI] [PubMed] [Google Scholar]
- Ricken AM, Traenkner A, Merkwitz C, Hummitzsch K, Grosche J, Spanel-Borowski K. The short prolactin receptor predominates in endothelial cells of micro-and macrovascular origin. J Vasc Res. 2007;44:19–30. doi: 10.1159/000097892. [DOI] [PubMed] [Google Scholar]
- Chiwororo WD, Ojewole JA. Biphasic effect of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract on rat isolated vascular smooth muscles. J Smooth Muscle Res. 2008;44:217–29. doi: 10.1540/jsmr.44.217. [DOI] [PubMed] [Google Scholar]
- Wong ES, Man RY, Vanhoutte PM, Ng KF. Dexmedetomidine induces both relaxations and contractions, via different {alpha}2-adrenoceptor subtypes, in the isolated mesenteric artery and aorta of the rat. J Pharmacol Exp Ther. 2010;335:659–64. doi: 10.1124/jpet.110.170688. [DOI] [PubMed] [Google Scholar]
- Castillo-Hernandez J, Torres-Tirado D, Barajas-Espinosa A, Chi-Ahumada E, Ramiro-Díaz J, Ceballos G, et al. Two dissimilar AT1 agonists distinctively activate AT1 receptors located on the luminal membrane of coronary endothelium. Vascul Pharmacol. 2009;51:314–22. doi: 10.1016/j.vph.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Ishihata A, Endoh M. Species-related differences in inotropic effects of angiotensin II in mammalian ventricular muscle: receptors, subtypes and phosphoinositide hydrolysis. Br J Pharmacol. 1995;114:447–53. doi: 10.1111/j.1476-5381.1995.tb13247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch-Weser J. Nature of the inotropic action of angiotensin on ventricular myocardium. Circ Res. 1965;16:230–7. doi: 10.1161/01.res.16.3.230. [DOI] [PubMed] [Google Scholar]
- Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ Res. 2006;99:570–82. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- Firdolas F, Ogras MS, Ozan T, Onur R, Orhan I. In vitro examination of effects of hyperprolactinemia and hypoprolactinemia on seminal vesicle contraction. Urology. 2013;81:557–61. doi: 10.1016/j.urology.2012.11.025. [DOI] [PubMed] [Google Scholar]
- Georgiopoulos GA, Stamatelopoulos KS, Lambrinoudaki I, Lykka M, Kyrkou K, Rizos D, et al. Prolactin and preclinical atherosclerosis in menopausal women with cardiovascular risk factors. Hypertension. 2009;54:98–105. doi: 10.1161/HYPERTENSIONAHA.109.132100. [DOI] [PubMed] [Google Scholar]