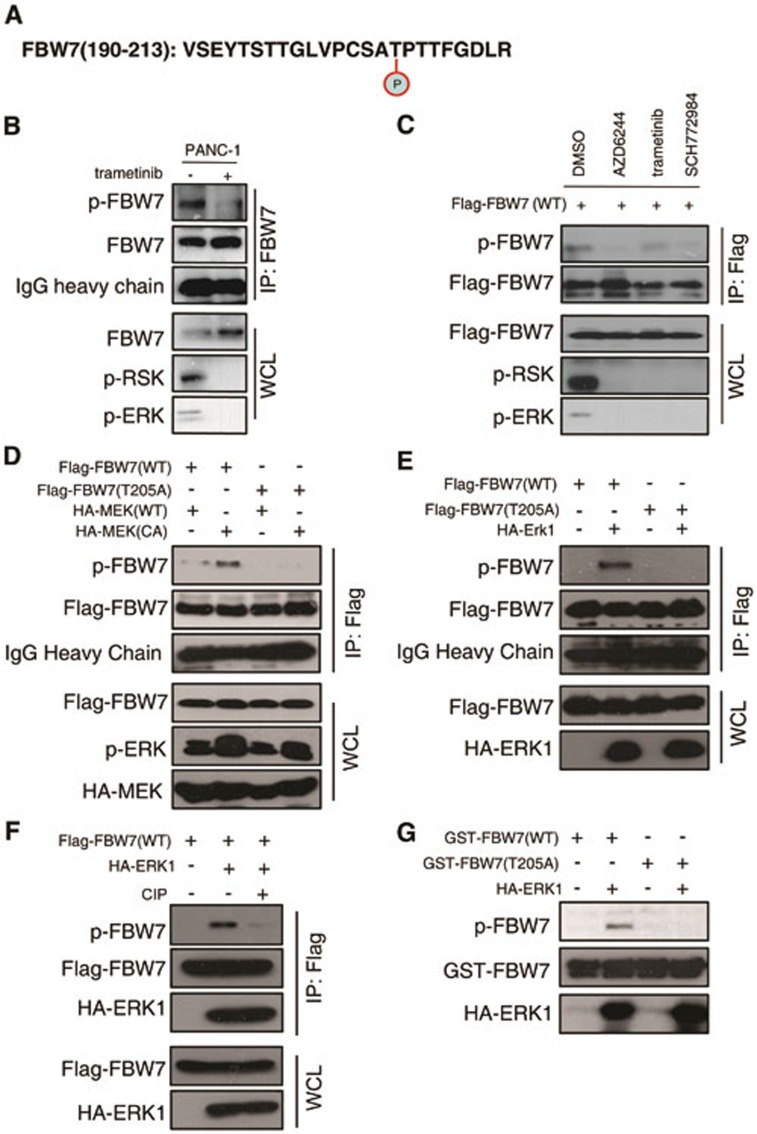
Figure 5.
ERK phosphorylates FBW7 at T205. (A) Detection of in vivo FBW7 T205 phosphorylation by mass spectrometry analysis. (B) PANC-1 cells were pretreated with the proteasome inhibitor MG132 and trametinib, as indicated, overnight before harvest. Endogenous FBW7 phosphorylation status was examined by immunoblot analysis after immunoprecipitates (IP). (C) 293T cells were transfected with Flag-WT-FBW7. Thirty hours posttransfection, cells were pretreated with MG132 and various MEK/ERK inhibitors overnight before harvesting. FBW7 phosphorylation status was examined by immunoblot analysis after immunoprecipitation. (D-F) 293T cells were transfected with Flag-WT-FBW7 and Flag-T205A-FBW7, together with HA-MEK1 and HA-ERK1 constructs, respectively. Wild-type (WT) and the constitutively active (CA) form of MEK1 were used as indicated. Thirty hours post-transfection, cells were pretreated with MG132 overnight before harvesting. FBW7 phosphorylation status was examined by immunoblot analysis after immunoprecipitation. Immunoprecipitates were treated with CIP for 20 min at room temperature, and the reaction was stopped by the addition of SDS loading buffer (F). (G) Recombinant GST-WT-FBW7 and GST-T205A-FBW7 proteins were incubated with HA-ERK1 kinase in the presence of ATP and the kinase reaction buffer. Thirty minutes later, the reaction was stopped by the addition of the loading buffer. The kinase reaction products were resolved by SDS-PAGE, and FBW7 phosphorylation was detected by the specific p-FBW7 antibody.