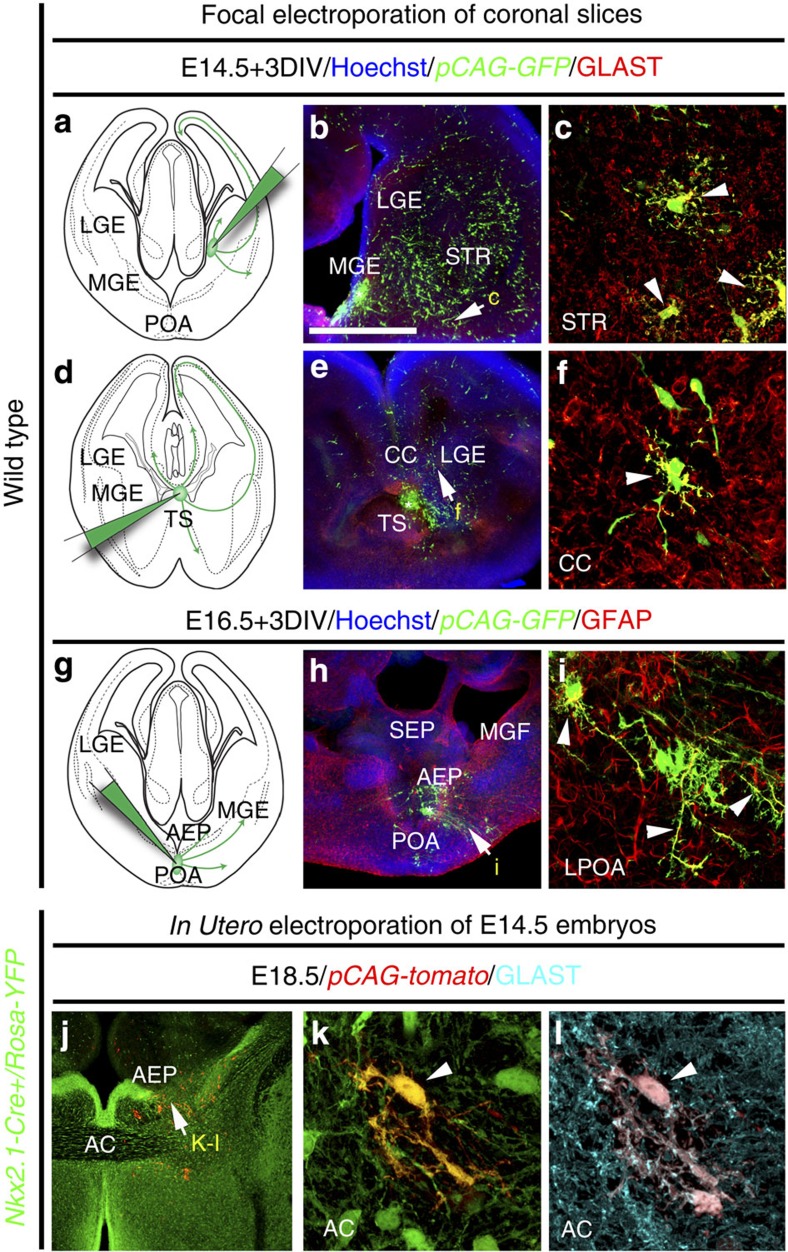
Figure 4. Nkx2.1-derived subpallial domains give rise to embryonic astroglia.
(a–i) Use of in vitro electroporation to determine whether embryonic astrocytes originate from subpallial sites. The MGE (n=12; a–c), TS (n=7; d–f) and the POA (n=6; g–i) of WT E14.5–E16.5 coronal telencephalic slices were focally electroporated with a plasmid (pCAG-EGFP) expressing the EGFP. Double immunochemistry for GFP and GLAST (b,c,e,f), and for GFP and GFAP (h,i) was performed. (c,f,i) High-magnified views of the regions indicated by an arrow in b,e,h, respectively. (a–f) After focal electroporation, subpallial progenitors gave rise to GFP+/GLAST+ astrocytes that have migrated in the striatal mantle (STR) and the CC (solid arrowheads in c,f). (g–i) A similar electroporation into the POA, gave rise to GFP+/GFAP+ astroglia that have migrated to the lateral part of the POA (LPOA; solid arrowheads in i). (j–l) Experimental paradigms used to ascertain the Nkx2.1-derived origin of embryonic astroglia. To this end, a Tomato expressing plasmid (pCAG-tomato) was electroporated into the subpallial domain of Nkx2.1-Cre+/Rosa-YFP embryos at E14.5 and triple immunohistochemistry for YFP, tomato and GLAST was performed (n=8). (j–l) After in utero electroporation, progenitors of the AEP gave rise to several YFP+ Nkx2.1-derived astroglia, labelled for tomato and GLAST, that migrate to the AC. Cell nuclei were counterstained in blue with Hoechst (b,e,h). Colocalization between the green and the red channel is highlighted in yellow (c,f,i,k). The scale bar shown in panel a corresponds to the following length for panel(s) specified in brackets: 675 μm (b,e,h,j); 60 μm (c,f,i); 40 μm (k,l).